Methods for treating metabolic disorders and obesity with gip and glp-1 receptor-active glucagon-based peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Extended Half-Life GLP-1 / GIP Coagonist Peptide was Well Tolerated with an Extended Half-Life of about 5 Days
[0152]A Phase I, randomized, placebo-controlled, sequential, single-ascending, two period, study was performed in healthy male subjects to evaluate the safety, tolerability, pharmacokinetic (PK), and pharmacodynamic (PD) properties of extended half-life GLP-1 / GIP coagonist peptide. Healthy adult male subjects (about 18 to 55 years of age) who were in general good health based on medical history, physical examination, ECG, and routine laboratory tests (e.g., blood, chemistry, hematology, urinalysis, and drug screen) with a body mass index between 20 kg / m2 and 30 kg / m2 were selected for the study. The extended half-life GLP-1 / GIP coagonist peptide tested in all of the Examples was SEQ ID NO: 153.
[0153]The subjects were randomized into cohorts. Cohort 1, 2, 3, 4, 5, and 6 each consisted of 6 patients that received 0.1 mg, 0.3 mg, 1 mg, 2 mg, 4 mg, and 8 mg, respectively of the ex...
example 2
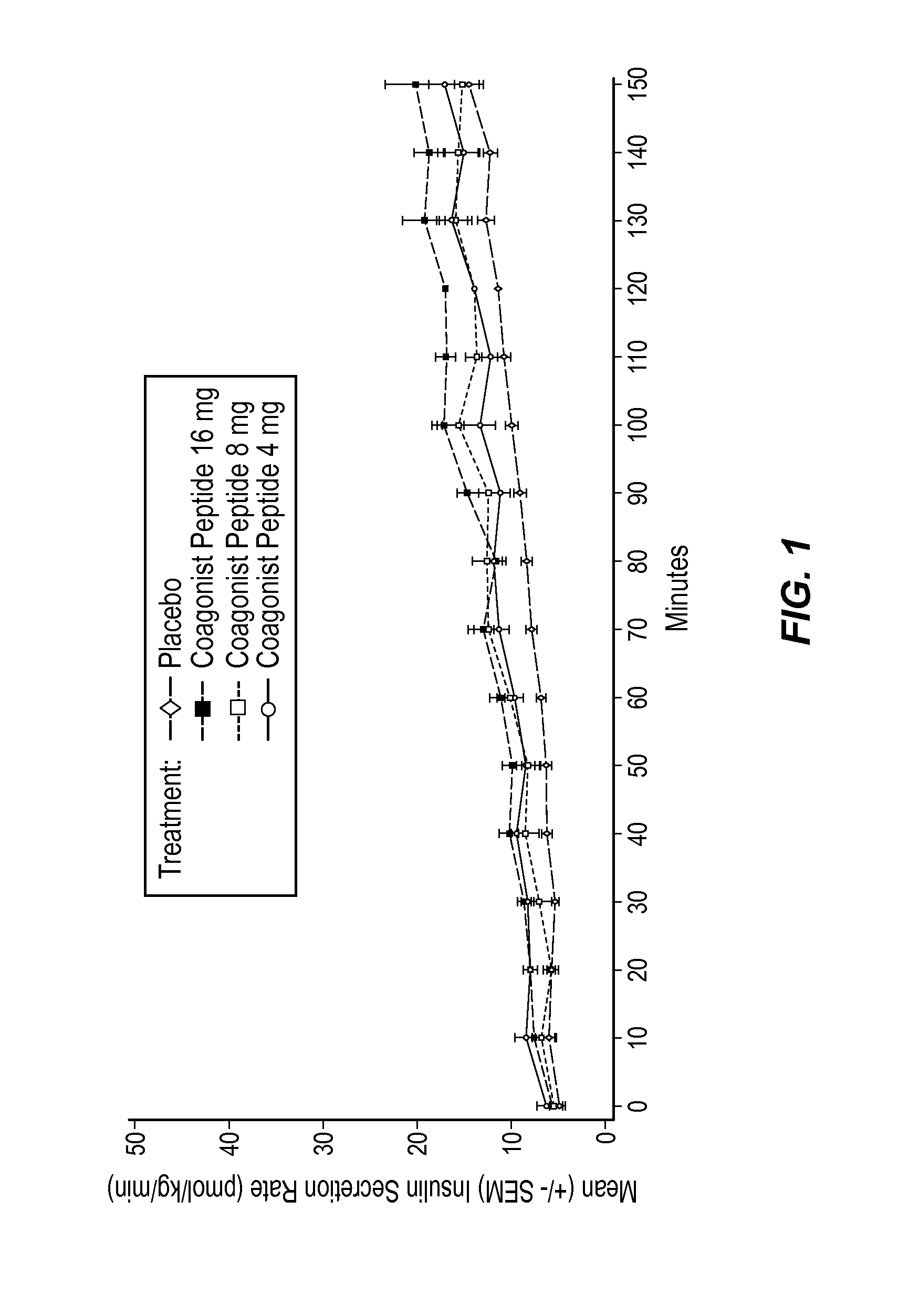
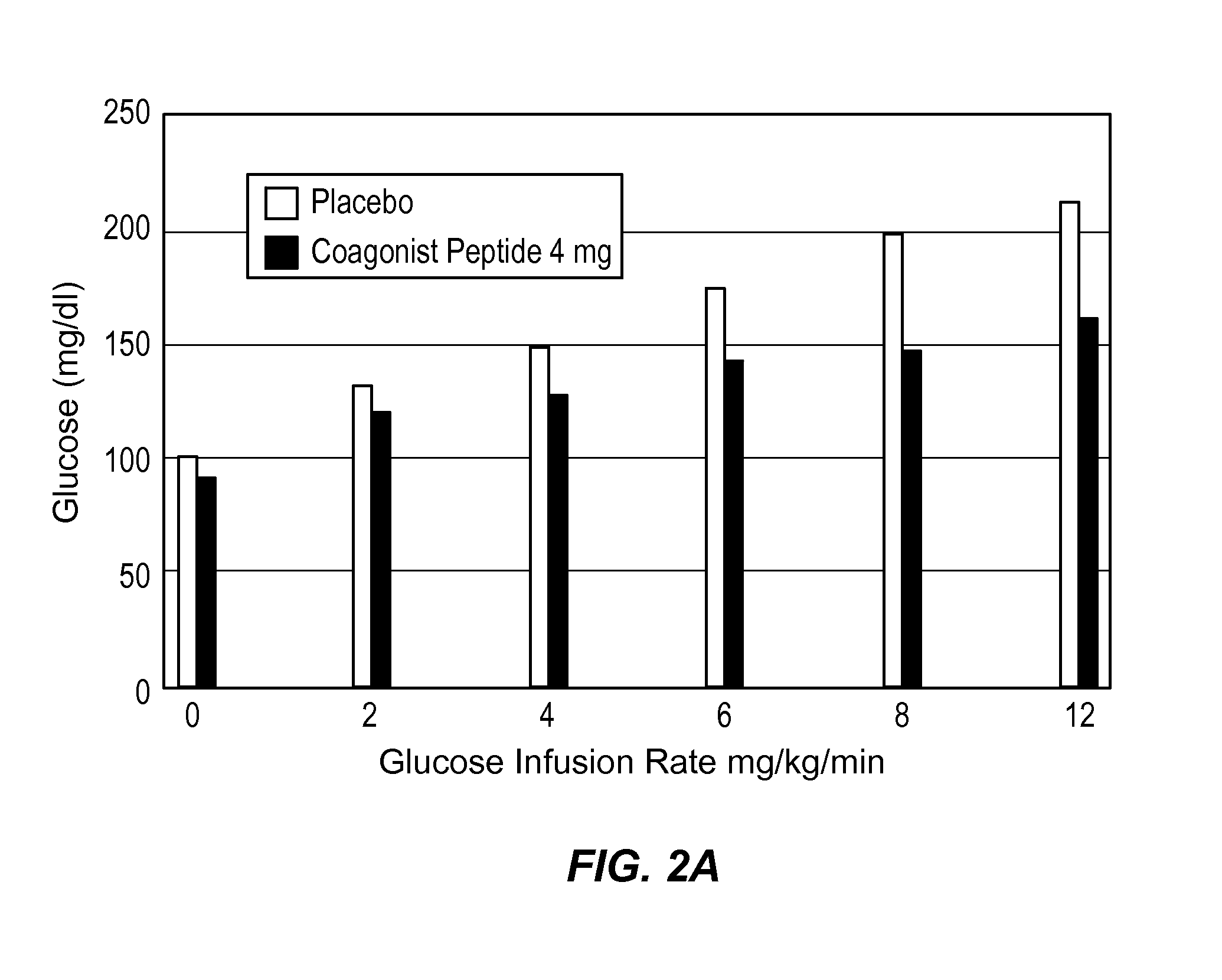
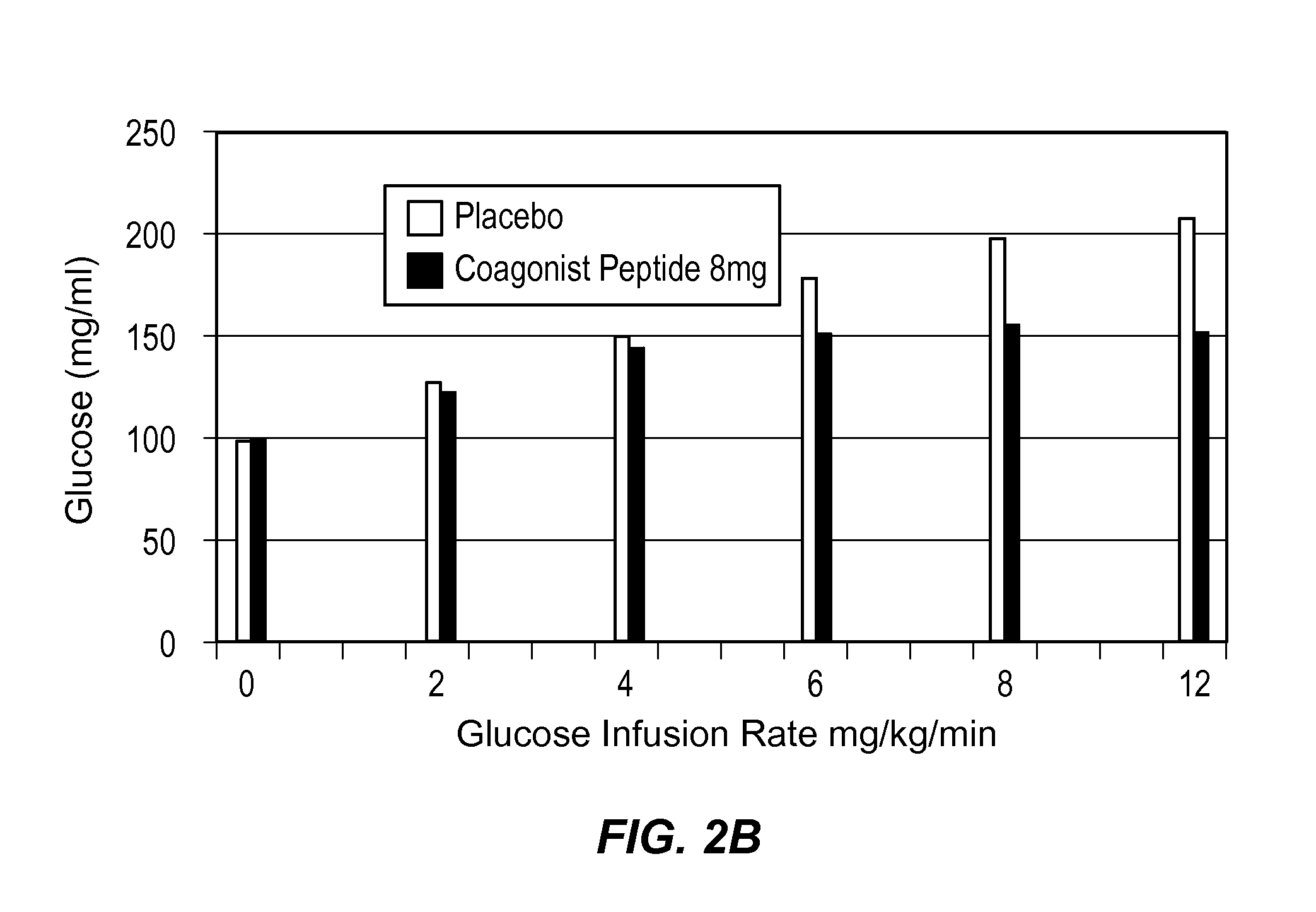
Extended Half-Life GLP-1 / GIP Coagonist Peptide Increased Insulin Secretion in a Dose-Dependent Manner
[0159]A Phase I, randomized, placebo-controlled, positive-controlled two-part study was performed in healthy male and female subjects to evaluate the effect of a range of doses on beta cell response to a glucose load and to assess the effect of these doses on gastric emptying. Beta cell function was assessed by calculation of prehepatic insulin secretion in response to a graded glucose infusion. Gastric emptying was assessed by measurement of plasma appearance of ingested acetaminophen.
[0160]Subjects in Part 1 (Cohort 1) received two subcutaneous (SC) injections of placebo, 2 hours apart, in the abdomen on Day 1, followed by two 5 μg subcutaneous (SC) injections of BYETTA (Amylin Pharmaceuticals), 2 hours apart (10 μg total) in the abdomen on Day 2. During Part 2, subjects in Cohort 2 received placebo on Day 1, followed by 8 mg of the extended half-life GLP-1 / GIP coagonist peptide on...
example 3
Effect of Extended Half-Life GLP-1 / GIP Coagonist Peptide in Patients with Type II Diabetes Mellitus
[0177]A randomized, placebo-controlled, sequential, multiple-ascending dose study is performed on male and female patients (18 to 70 years of age) diagnosed with type 2 diabetes and on stable metformin monotherapy (i.e., at the same dose for at least about 2 months prior to the study), with an HbA1c level of at least about 6.5% and no more than 10.5%. Other criteria include fasting glucose levels of 110 mg / dL to 200 mg / dL, body mass index of 27 kg / m2 to 40 kg / m2, systolic blood pressure less than 155 mmHg, diastolic blood pressure less than 95 mmHg and no history of significant other disease or complications.
[0178]The patients are randomized into 4 cohorts. Cohort 1 consists of 8 patients that receive 4 mg of the extended half-life GLP-1 / GIP coagonist peptide and 2 patients that receive placebo. Cohort 2 consists of 8 patients that receive 12 mg of the extended half-life GLP-1 / GIP coag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com