Methods for reducing matrix-bound nicotine-derived nitrosamine ketone in tobacco plant material
a technology of matrix-bound nitrosamine and ketone, which is applied in the field of methods for reducing the amount of matrix-bound nitrosamine ketone or 4(methylnitrosamino)1(3pyridyl)1butanone (nnk) in tobacco plant material, can solve the problems of difficult removal or extraction, unsuitable use of tobacco stems and tobacco fines from manufacturing processes, and deterioration of tobacco quality, so as to reduce the amount of matrix-bound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method for Analysis of Free and Matrix-Bound NNK in Tobacco
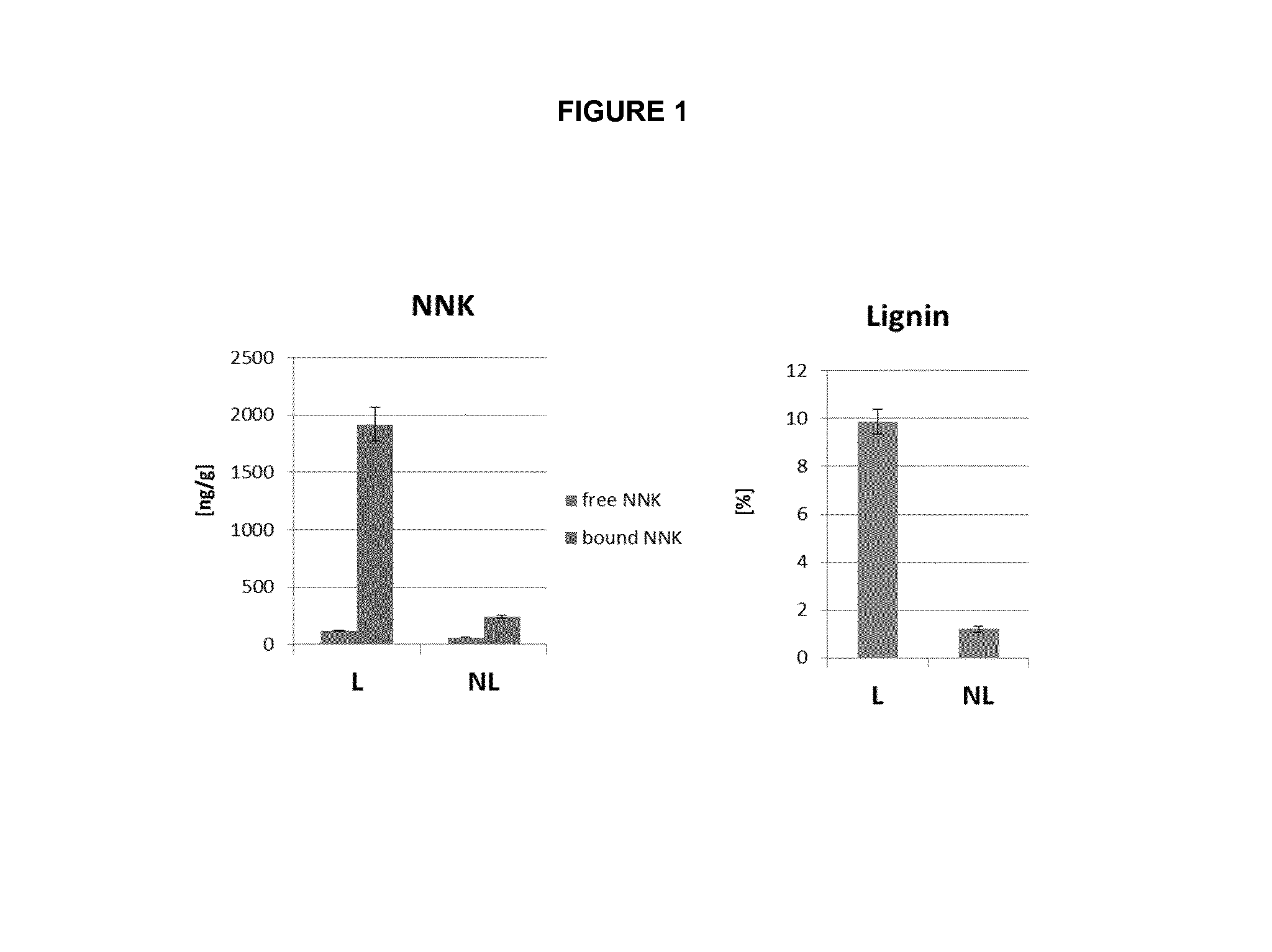
[0116]Aliquots of tobacco samples (for example, about 750 mg) are extracted with about 30 mL of Tris-HCl buffer (50 mM; pH 7.4) by shaking for about one hour at approximately room temperature. Internals standard (100 ng / mL NNK-d4) are added. Samples (0.4 mL) of the extracts are filtered using a 0.2 μM filter and the NNK content is analysed using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS). The sample concentrations calculated from these extract concentrations correspond to the “free NNK” concentrations in the sample. After treating the extraction mixtures (for example, by heating to about 130° C. for about 4 hours) and filtering aliquots of the extracts, NNK concentrations are again measured by UPLC-MS / MS. From these values, the “total NNK” concentration in the samples can be calculated. The “matrix-bound NNK” concentration is the difference between the “total NNK” and the “free NNK” concent...
example 2
UPLC Analysis
[0118]The column used is Waters Acquity BEH C18, 1.7 μm, 2.1×50 mm. The eluents used are: (A) ammonium bicarbonate (10 mM; adjusted to pH 9.8 with ammonia)+2% (v / v) acetonitrile; (B) acetonitrile. The gradient used is 0 min—5% B; 0.5 min—5% B; 3.3 min—18.3% B. The flow that is used is 0.5 mL / min. The column temperature that is used is 50° C.
example 3
MS / MS Methodology
[0119]This analysis is carried out on a Waters TQ spectrometer using the following MRM transitions: NNK: 208.2→122.2; dwell time 100 ms; NNK-d4: 212.2→126.2; dwell time 100 ms; Capillary voltage: 0.6 kV; Cone voltage: 25 V; Collision energy: 11 eV; Source temperature: 120° C.; Desolvation temperature: 400° C.; Desolvation gas flow: 800 L / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com