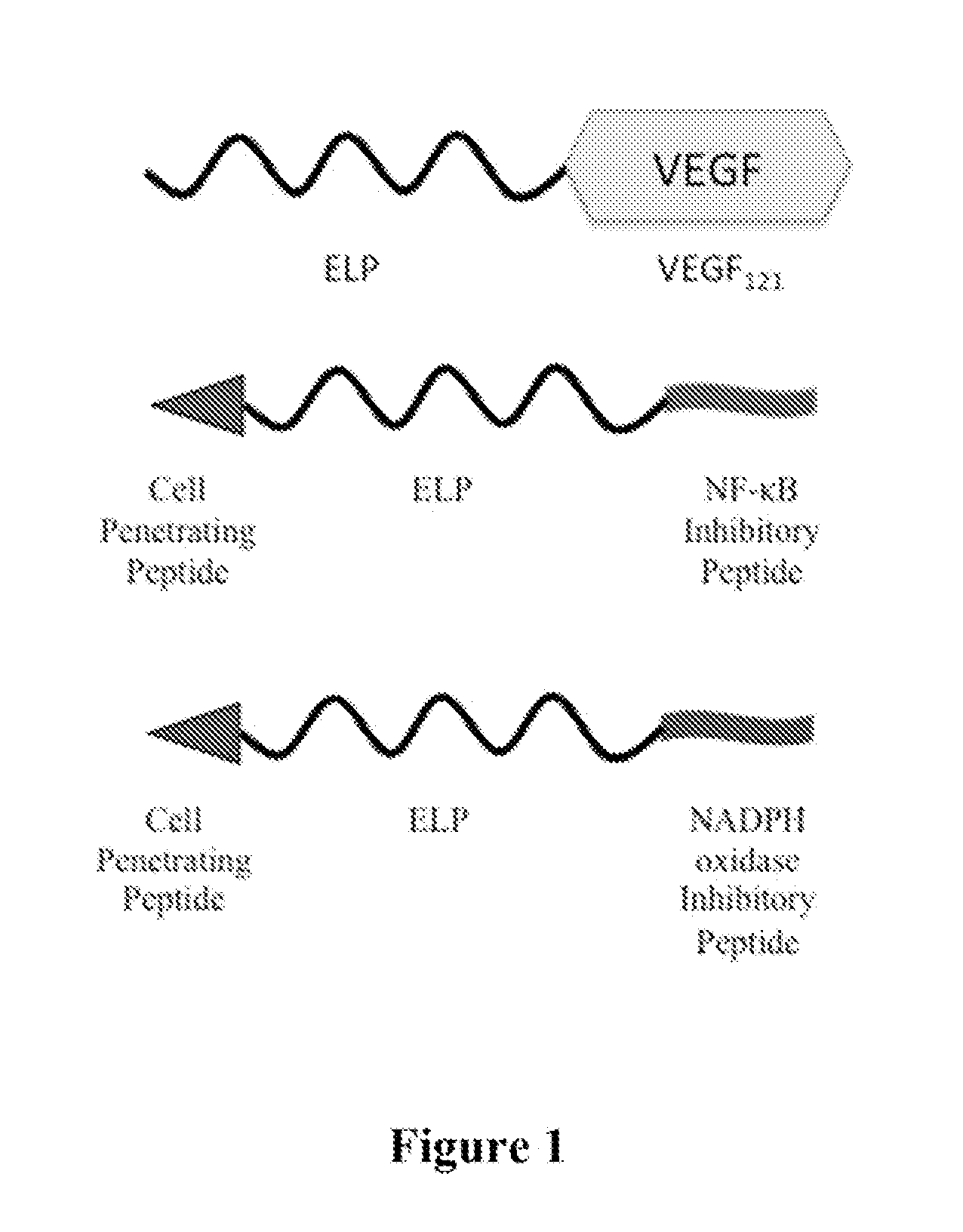
Composition and Method for Therapeutic Agent Delivery During Pregnancy
a technology of therapeutic agents and delivery methods, applied in the direction of peptide/protein ingredients, fusions for specific cell targeting, peptide sources, etc., can solve the problems of severe adverse effects on the developing fetus, and achieve the effect of reducing the amount of therapeutic agents that cross the placenta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design and Method for Generation of ELP-Fusion Polypeptides
[0088]ELP sequences were made by recursive directional ligation. A synthetic nucleotide cassette containing the coding sequence for 5 to 10 VPGXG repeats with the desired amino acids at the X position and flanked by PflMI and BlgI restriction sites was cloned into the pUC19 vector at the EcoRI and HinDIII sites. The sequence of this construct was confirmed by DNA sequencing using standard M13 forward and reverse primers. Once one block of 5 to 10 VPGXG repeats was inserted and confirmed, it was excised from pUC19 using PflMI and BglI restriction digestion and purified using agarose electrophoresis. A second aliquot of pUC19 containing the VPGXG repeated sequence was linearized by digestion with PflMI only, and the gel purified cassette was ligated into the PflMI restriction site. This resulted in an in-frame fusion of the block of 5-10 VPGXG repeats with a second block of 5 to 10 VPGXG repeats, effectively doubling the numbe...
example 2
Recombinant Expression and Purification of Polypeptides
[0090]ELPs and ELP fusion proteins were expressed and purified from E. coli BLR (DE3) or Rosetta2®(DE3) (for constructs resulting from human cDNA containing human-optimized codons). Briefly, 500 mL of TB Dry liquid culture media (MoBio) was inoculated with the expression strain and cultured at 37° C. with 250 rpm agitation for 16-18 h. In the absence of the pLysS lysozyme-expressing plasmid, the pET expression system allows for leaky production of the recombinant protein even without inducing agents. Bacteria were harvested by centrifugation and lysed by sonication (10×10 sec pulses, 75% amplitude, Fisher Sonic Dismembrator). Cell debris was removed by centrifugation, and nucleic acids were precipitated with 10% polyethylene imine and removed by centrifugation. NACl was added to the soluble bacterial lysate to lower the ELP transition temperature (4 g / 30 mL), and the lysate was heated to 42° C. to induce aggregation of the ELP-c...
example 3
Use of ELP for Drug Delivery During Pregnancy
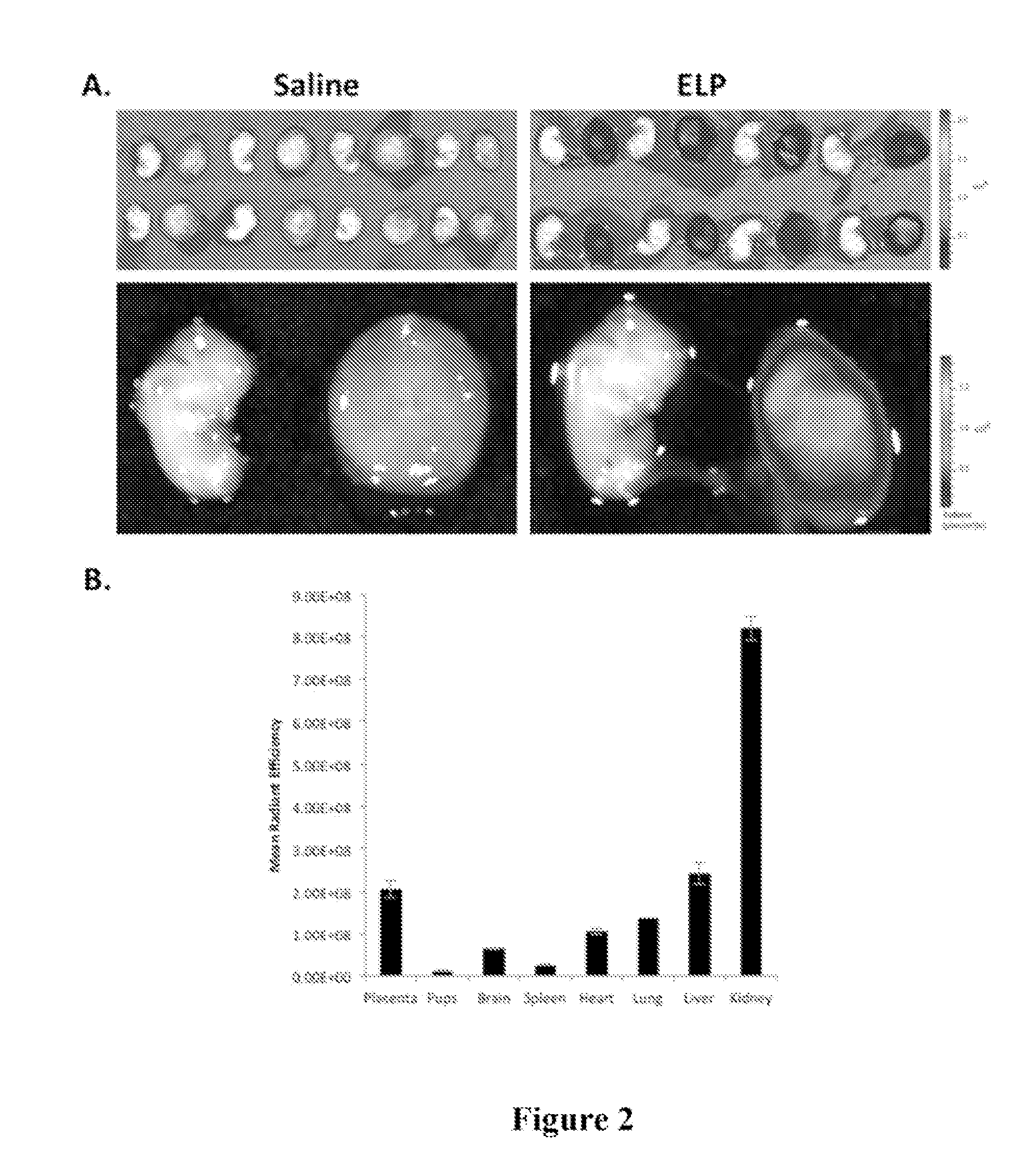
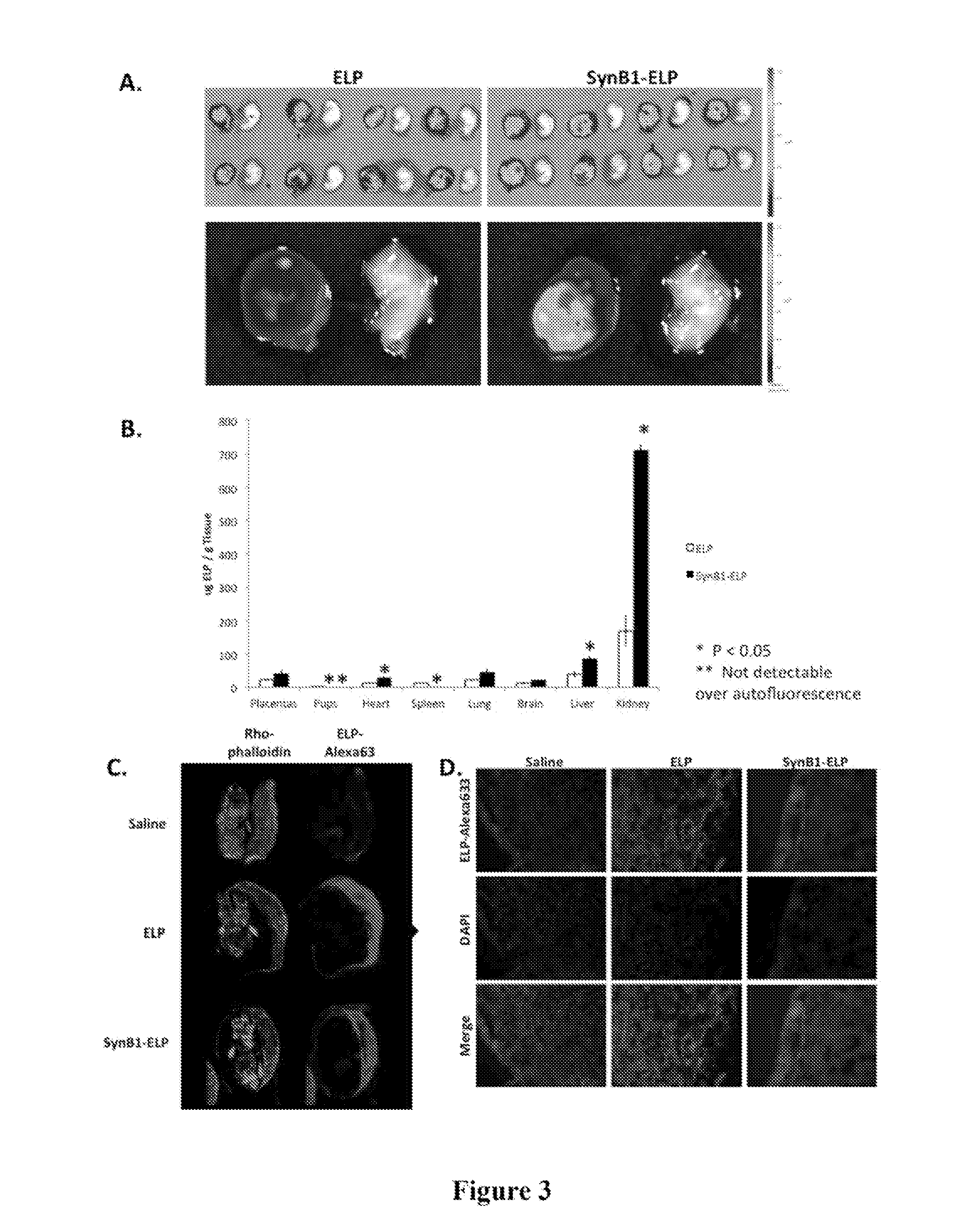
[0091]In order to test the hypothesis that ELP-fused therapeutics do not cross the placenta, an experiment was performed using the unmodified ELP carrier. Pregnant Sprague Dawley rats on day 14 of gestation were injected with fluorescently labeled ELP (100 mg / kg IV). Four hours after injection, which is about one half-life for this polypeptide, the rats were sacrificed and the placentas, pups, and major organs were removed for examination. Placentas and pups were dissected from the amniotic sacks and imaged ex vivo using an IVIS Spectrum animal imager to detect and quantitate the ELP levels. As shown in FIG. 2A, the placentas of animals injected with ELP-Alexa633 stained brightly, indicating that much protein had accumulated in them. In contrast, almost no ELP was detectable in the pups. The image intensities of the placentas, pups, and major organs were quantitated using Living Image software, and the results are shown in FIG. 2B. ELP ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com