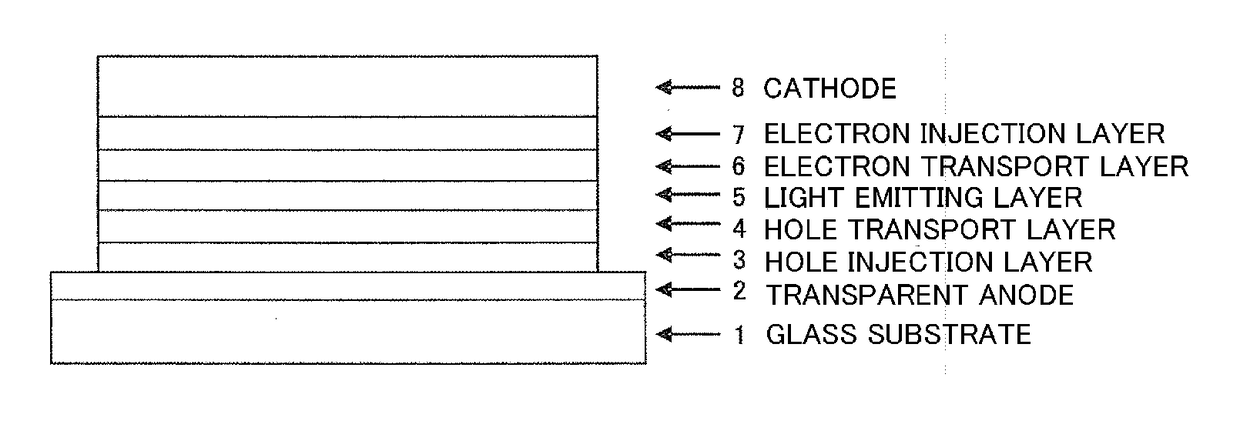
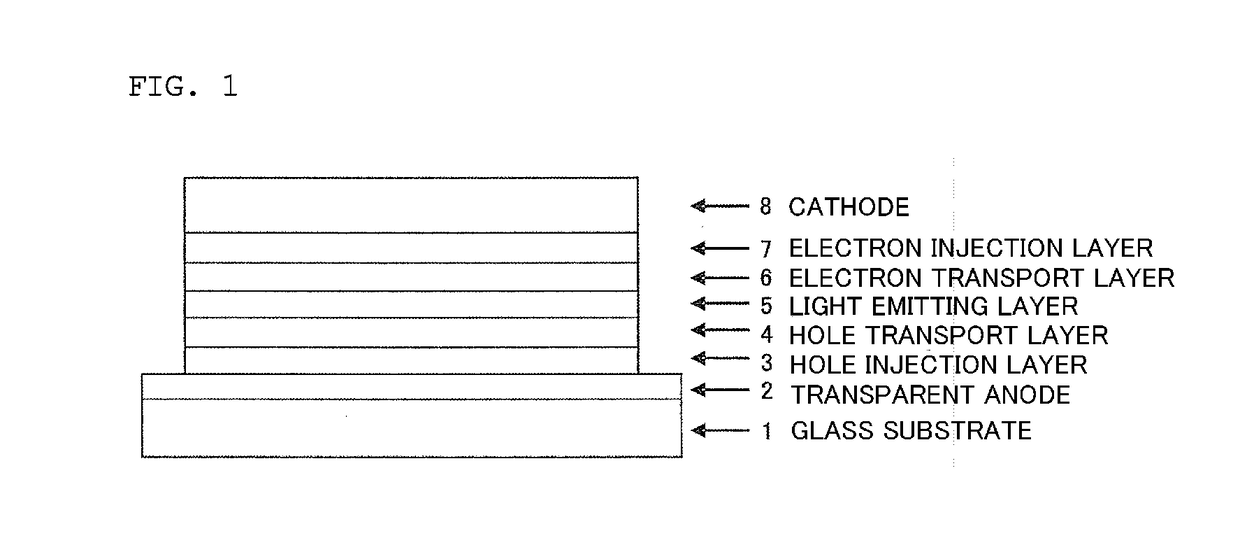
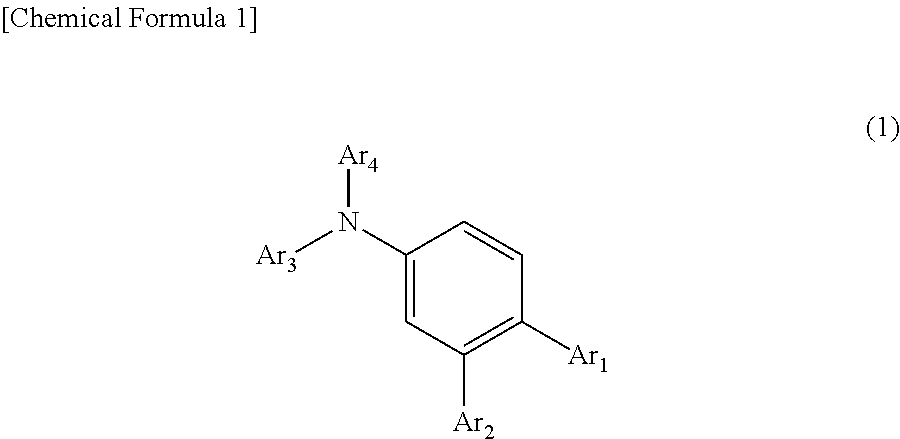
Organic electroluminescent device
an electroluminescent device and organic technology, applied in the field of organic electroluminescent devices, can solve the problems of deterioration of the device, inability to achieve improvement in luminous efficiency, and material degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of N,N-bis(biphenyl-4-yl)-N-(6-phenylbiphenyl-3-yl)amine (Compound 1-2)
[0221]N,N-bis(biphenyl-4-yl)-N-(6-bromobiphenyl-3-yl)amine (11.8 g), toluene (94 mL), phenylboronic acid (2.7 g), and an aqueous solution obtained by previously dissolving potassium carbonate (5.9 g) in water (36 mL) were added into a nitrogen-substituted reaction vessel and aerated with nitrogen gas under ultrasonic irradiation for 30 minutes. Tetrakistriphenylphosphine palladium (0.74 g) was added thereto, and the resulting mixture was heated and stirred at 72° C. for 18 hours. After the mixture was cooled to a room temperature, an organic layer was collected by liquid separation. The organic layer was washed with water, and washed with a saturated salt solution sequentially, and then dried over anhydrous magnesium sulfate and concentrated to obtain a crude product. Subsequently, the crude product was purified using column chromatography, whereby a white powder of N,N-bis(biphenyl-4-yl)-N-(6-phenylbip...
example 2
Synthesis of N,N-bis(biphenyl-4-yl)-N-{6-(naphthyl-1-yl)biphenyl-3-yl}amine (Compound 1-3)
[0225]The reaction was carried out under the same conditions as those of Example 1, except that phenylboronic acid was replaced with 1-naphthylboronic acid, whereby a white powder of N,N-bis(biphenyl-4-yl)-N-{6-(naphthyl-1-yl)biphenyl-3-yl}amine (Compound 1-3, 9.2 g, yield: 61%) was obtained.
[0226]The structure of the obtained white powder was identified by NMR.
[0227]1H-NMR (CDCl3) detected 33 hydrogen signals, as follows.
[0228]δ (ppm)=7.84-7.87 (3H), 7.67-83 (6H), 7.26-7.64 (18H) 7.02-7.04 (6H)
example 3
Synthesis of N,N-bis(biphenyl-4-yl)-N-{6-(9,9-dimethylfluoren-2-yl)biphenyl-3-yl}amine (Compound 1-1)
[0229]The reaction was carried out under the same conditions as those of Example 1, except that phenylboronic acid was replaced with (9,9-dimethylfluoren-2-yl)boronic acid, whereby a white powder of N,N-bis(biphenyl-4-yl)-N-{6-(9,9-dimethylfluoren-2-yl)biphenyl-3-yl}amine (Compound 1-1, 9.0 g, yield: 57%) was obtained.
[0230]The structure of the obtained white powder was identified by NMR.
[0231]1H-NMR (CDCl3) detected 39 hydrogen signals, as follows.
[0232]δ (ppm)=7.56-7.64 (10H), 7.26-50 (18H), 7.02-7.16 (5H), 1.26 (6H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com