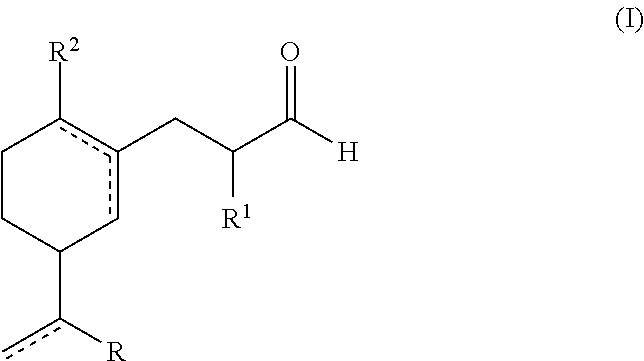
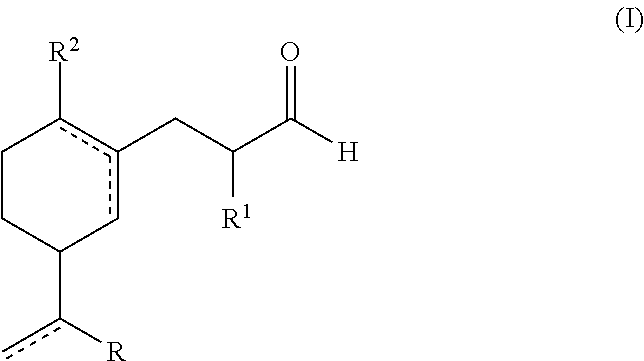
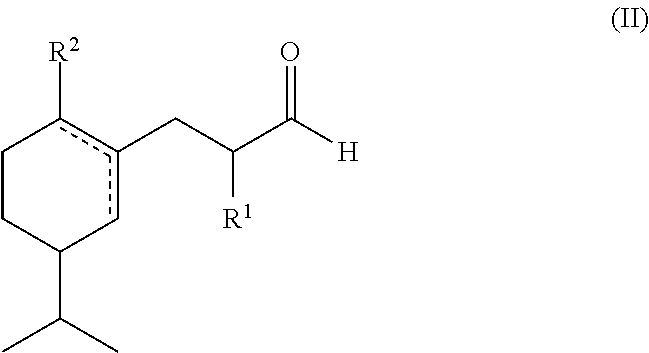
A green, lily of the valley perfuming ingredient
a perfuming ingredient and green lily of the valley technology, applied in the field of perfume, can solve the problems of no prior art document reporting or suggesting any organoleptic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compounds of Formula (I)
a) Preparation of a Mixtures of 3-[5-(2-propanyl)-1-cyclohexen-1-yl]propanal and its Isomer 3-[3-(2-propanyl)-1-cyclohexen-1-yl]propanal
i) 7-isopropyl-1-oxaspiro[4.5]decan-2-one
[0070]3-isopropyl-1-cyclohexanol (140 g; 984 mmol; 2 eq.) was heated in an oil bath at 155° C. under nitrogen. Di-tert-butyl peroxide (18.36 g; 123 mmol; 0.25 eq.) and n-butyl acrylate (63.7 g; 492 mmol; 1 eq.) were added simultaneously and separately over a period of 1 hour. After 4 more hours at 150° C., the reaction was cooled to 50° C. and MTBE (100 mL) was added, followed by 30% aqueous NaOH solution (118 g; 886 mmol). After stirring for 30 minutes, water (140 mL) was added, followed by MTBE (140 mL). The phases were separated and the aq. phase was treated with diethyl ether (2×300 mL) and MTBE (300 mL). Each organic phase was treated with water (210 mL). The combined aqueous phases were treated with 50% aqueous H2SO4 solution (280 g), extracted twice with diethyl eth...
example 2
Preparation of a Perfuming Composition
[0095]A perfuming composition, of the floral type, was prepared by admixing the following ingredients:
Parts byweightIngredient100Acropal 1)40C 12 Aldehyde10C 8 Aldehyde501%* Cuminic aldehyde20(3,7-Dimethyl-6-octenyloxy)acetaldehyde209-Undecenal1000Coranol ®2)300Hivernal ®3)10003-(4-Isopropylphenyl)-2-methylpropanal5000Florol ®4)2010%* (4-methylphenoxy)acetaldehyde500Lilyflore ®5)300Mayol ®6)500(4E)-4-methyl-5-(4-methylphenyl)-4-pentenal5003 -(4,4-dimethyl-1-cyclohexen-1-yl)propanal1401%* Trans Decenal1007-(2-methyl-2-propanyl)-2H-1,5-benzodioxepin-3(4H)-one9600*in dipropyleneglycol1) 3 / 4-(4-methyl-3-pentenyl)-3-cyclohexene-1-carbaldehyde a)2) 4-cyclohexyl-2-methyl-2-butanol a)3) 3-(3,3 / 1,1-dimethyl-5-indanyl)propanal a)4) tetrahydro-2-isobutyl-4-methyl-4(2H)-pyranol a)5) (2,5-dimethyl-2,3-dihydro-1H-inden-2-yl)methanol a)6) cis-4-(1methylethyl)-cyclohexanemethanol a)a) origin: Firmenich SA, Geneva, Switzerland
[0096]The addition of 400 parts by w...
example 3
Preparation of a Perfuming Composition
[0099]A perfume, of the herbaceous, floral lily of the valley type, was prepared by admixing the following ingredients:
Parts byweightIngredient200Hexyl acetate500Isobornyl acetate100Geranyl acetate100Phenylethyl acetate50Styrallyl acetate400Verdyl acetate500Hexylcinnamic aldehyde1002-Methyl undecanal50Allyl Amyl Glycolate50Methyl anthranilate40Methyl benzoate250Benzylacetone150(1-Methyl-2-phenyl)ethyl butanoate107-Isopropyl-2H,4H-1,5-benzodioxepin-3-one100Cetalox ®1)30Raspberry ketone50Citronellyl Nitrile250Coumarine30Damascone Alpha600Dihydromyrcenol40Ethylvanilline100Eugenol300Fructalate ®2)400Gamma Undecalactone200Geraniol500Habanolide ®3)400Hedione ®4)100Allyl heptanoate100Ionone Beta300Iralia ®5)500Iso E ® Super 6)100Lavandin Grosso essential oil201-(2,2,3,6-Tetramethyl-cyclohexyl)-3-hexanol a)30Methyl Phenylethyl Ether502-Ethyl methylbutyrate20Methylparacresol30Crystal Moss oil100Muscenone ® Delta 7)20Neobutenone ® Alpha 8)100Nirvanol ®9)2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Odor threshold (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com