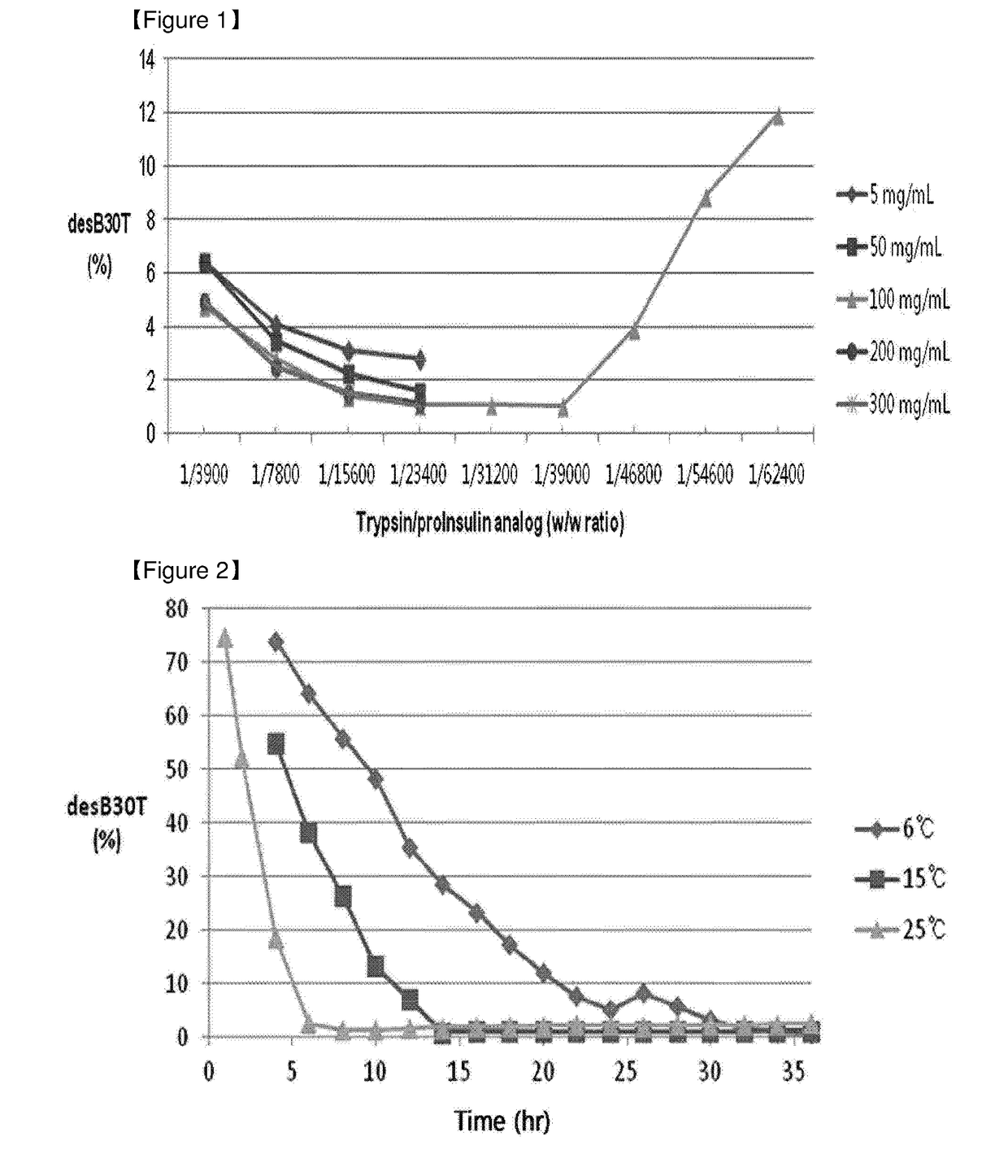
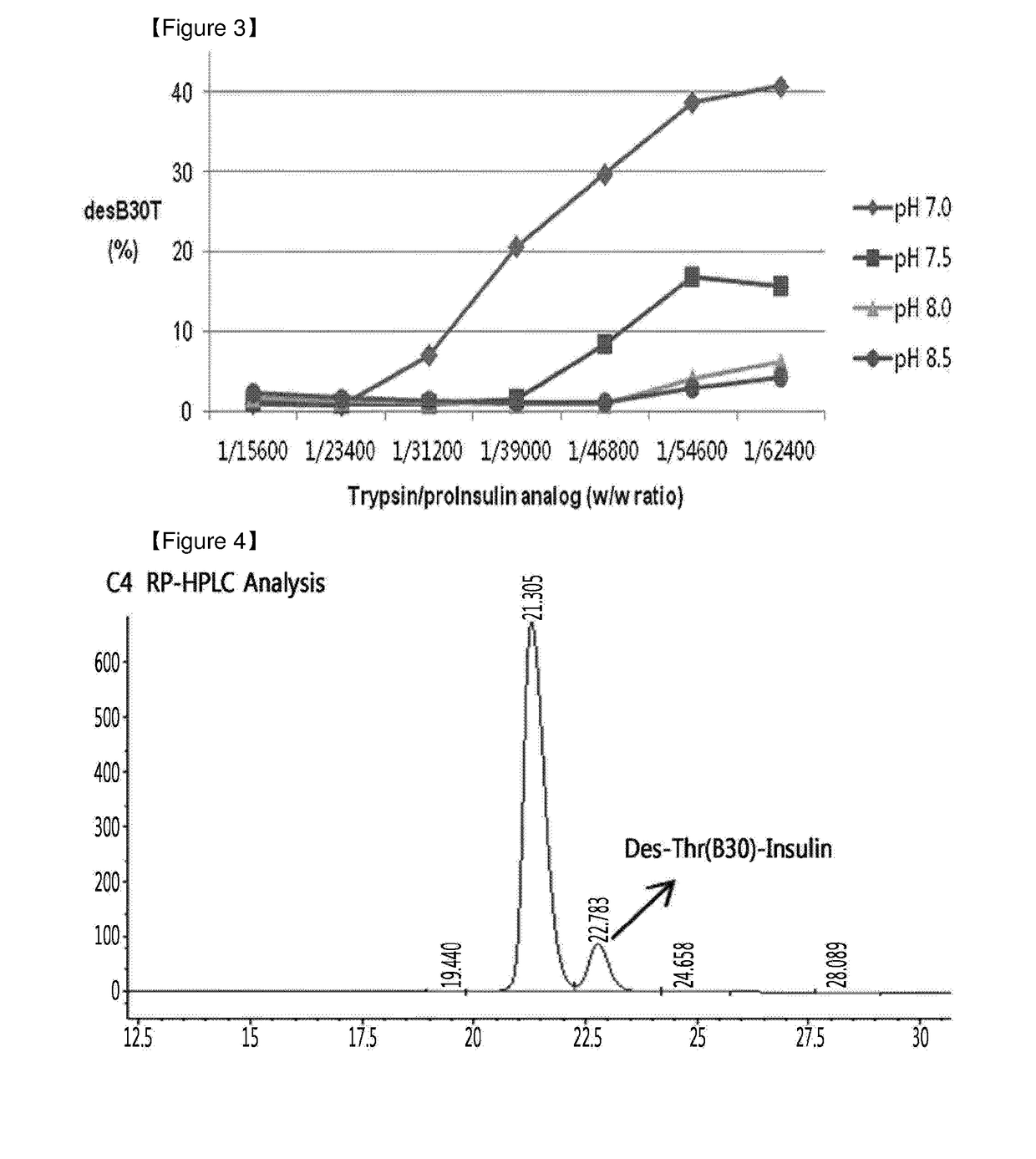
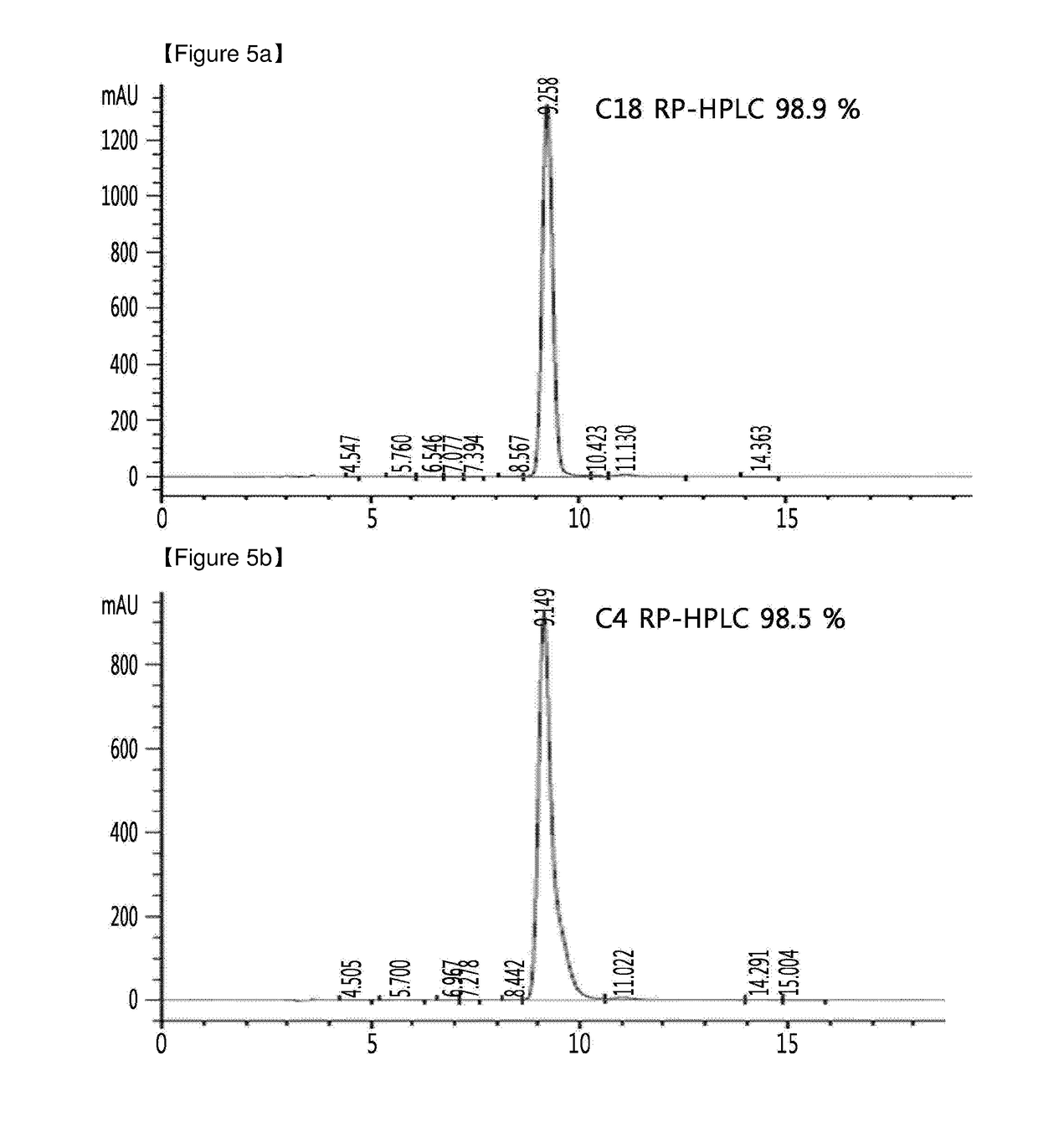
Method of insulin production
a technology of insulin and production method, which is applied in the field of preparing insulin, can solve the problems of increasing the procedural complexity and the increase in the production cost, and achieve the effects of reducing the cost of removing impurities, effective control, and improving the efficiency of insulin purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Proinsulin Analogs
[0139]The expression of recombinant proinsulin analogs was performed under the regulation of T7 promoter. The sequences corresponding to insulin of each analog are shown in Table 1 below.
TABLE 1 SEQ IDAnalogSequenceNOAnalog 1DNATTC GTT AAC CAA CAC TTG TGT GGC TCA CAC CTG GTG GAA GCT5CTC TAC CTA GTG TGC GGG GAA CGA GGC TTC TTC TAC ACA CCCAAG ACC CGC CGG GAG GCA GAG GAC CTG CAG GTG GGG CAG GTGGAG CTG GGC GGG GGC CCT GGT GCA GGC AGC CTG CAG CCC TTGGCC CTG GAG GGG TCC CTG CAG AAG CGT GCG ATT GTG GAA CAATGC TGT ACC AGC ATC TGC TCC CTC TAC CAG GTG GAG AAC TACTGC AAC Protein Phe Val Asn Gln His Leu Cys Gly Ser His Leu Val Glu Ala6Leu Tyr Leu Val Cys Gly Glu Arg Gly Phe Phe Tyr Thr ProLys Tnr Arg Arg Glu Ala Glu Asp Leu Gln Val Gly Gln ValGlu Leu Gly Gly Gly Pro Gly Ala Gly Ser Leu Gln Pro LeuAla Leu Glu Gly Ser Leu Gln Lys Arg Ala Ile Val Glu GlnCys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn TyrCys Asn Analog 2DNATTC GTT AAC CAA CAC TTG TGT GGC TCA CAC CTG GTG G...
example 2
and Refolding of Recombinant Proinsulin Analogs
[0142]In order to change the recombinant proinsulin analogs expressed in Example 1 to a soluble form, the cells were crushed and the analogs were refolded. The cell pellets, in the amount of 170 g (wet weight), were respectively resuspended in 1 L of a solubilizing buffer solution (50 mM Tris-HCl (pH 9.0), 1 mM EDTA (pH 8.0), 0.2 M NaCl, and 0.5% Triton X-100). The cells were crushed using M-110EH (Model M1475C, AC Technology Corp.), a microfluidizer processor, under a pressure of 15,000 psi. The crushed cell lysates were centrifuged at 4° C. at 12,000 g for 30 minutes and the supernatant discarded, and the pellets were respectively resuspended in 1 L of a washing buffer solution (0.5% Triton X-100, 50 mM Tris-HCl (pH 8.0), 0.2 M NaCl, and 1 mM EDTA). The resultants were centrifuged at 4° C. at 12,000 g for 30 minutes and the pellets were respectively resuspended in distilled water, and centrifuged in the same manner. The pellets were c...
example 3
ion by Cation Exchange Chromatography
[0143]A sample where the refolding was completed was attached to an SP-FF (GE healthcare, USA) column, which was equilibrated using a 20 mM sodium citrate (pH 3.0) buffer solution containing ethanol, and then the proinsulin analog protein was eluted by a linear concentration gradient from 0% to 100% using a 20 mM sodium citrate (pH 3.0) buffer solution containing 0.5 mM potassium chloride and ethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com