Direct NOX decomposition catalyst with improved activity and selectivity
a technology of direct decomposition and catalyst, which is applied in the field of catalysts, can solve the problems of low activity and/or selectivity of catalysts for direct decomposition, and the inability to produce desirable products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Examples 1-4 and the Comparative Example
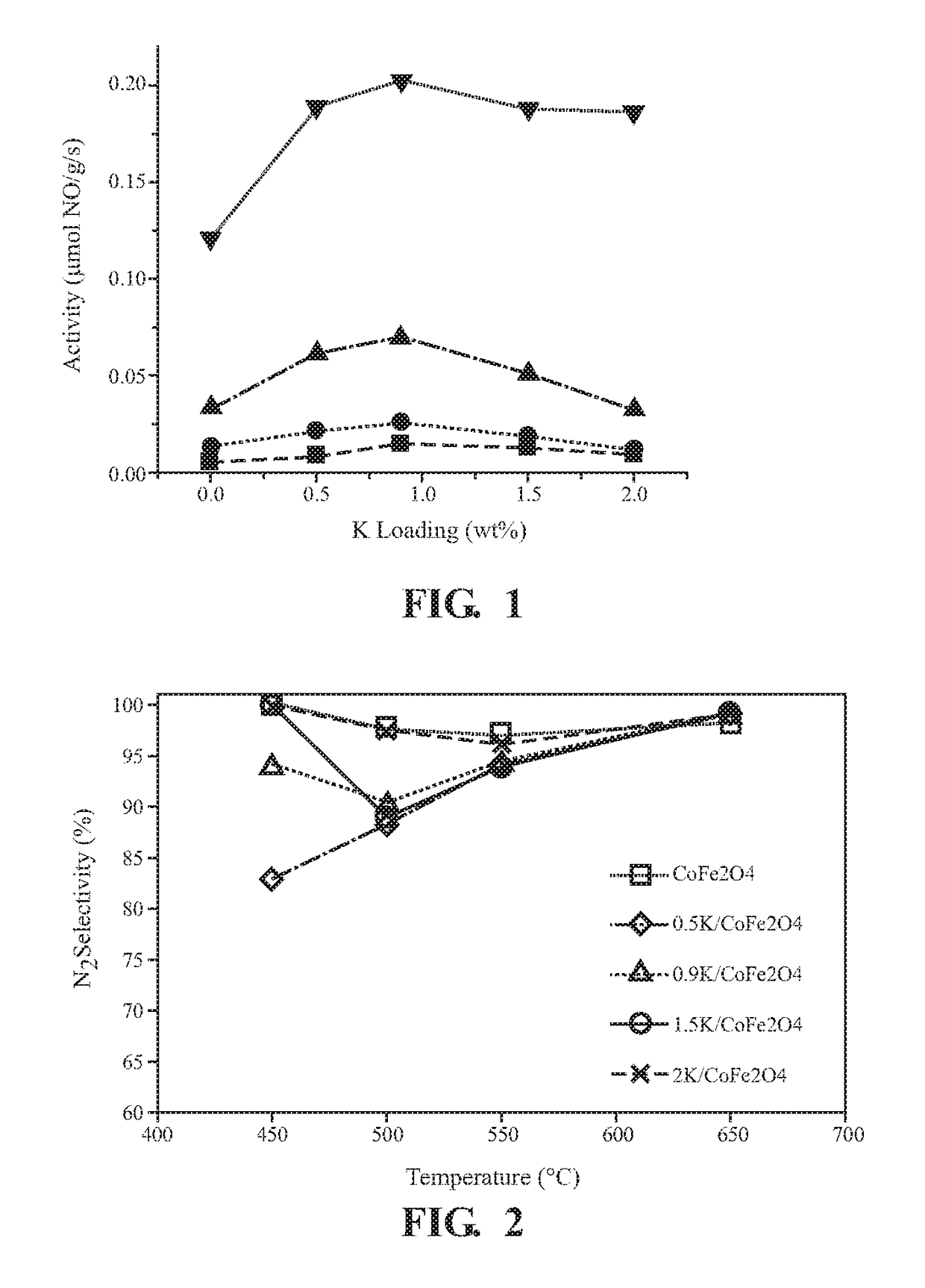
[0048]CoFe2O4 is purchased from Sigma Aldrich and calcined at 400° C. for 1 hour. Examples 1-4 and the Comparative Example are synthesized by a wet impregnation synthesis procedure. In a wet impregnation, 5 g of CoFe2O4 are mixed with and suspended in 50 mL of water. A calculated quantity of potassium hydroxide, as shown below in Table 1, is dissolved separately in deionized water and combined with the CoFe2O4 suspension, and the mixture is heated to 80° C. with continuous stirring. The resulting powder is then dried in an oven at 120° C. for 12 hours under air. Finally, the catalyst is calcined at 400° C. for 1 hour in the presence of air after ramping up to 400° C. with a 1° C. / min ramp.
TABLE 1Composition of wet impregnation solutionsWeight %MassMassPotassium inCoFe2O4KOHdoped catalystComparative5 grams0mg0.0ExampleExample 15 grams36.06mg0.5Example 25 grams65.16mg0.9Example 35 grams108.2mg1.5Example 45 grams146.4mg2.0
example 2
Characterization of Examples 1-4 and the Comparative Example
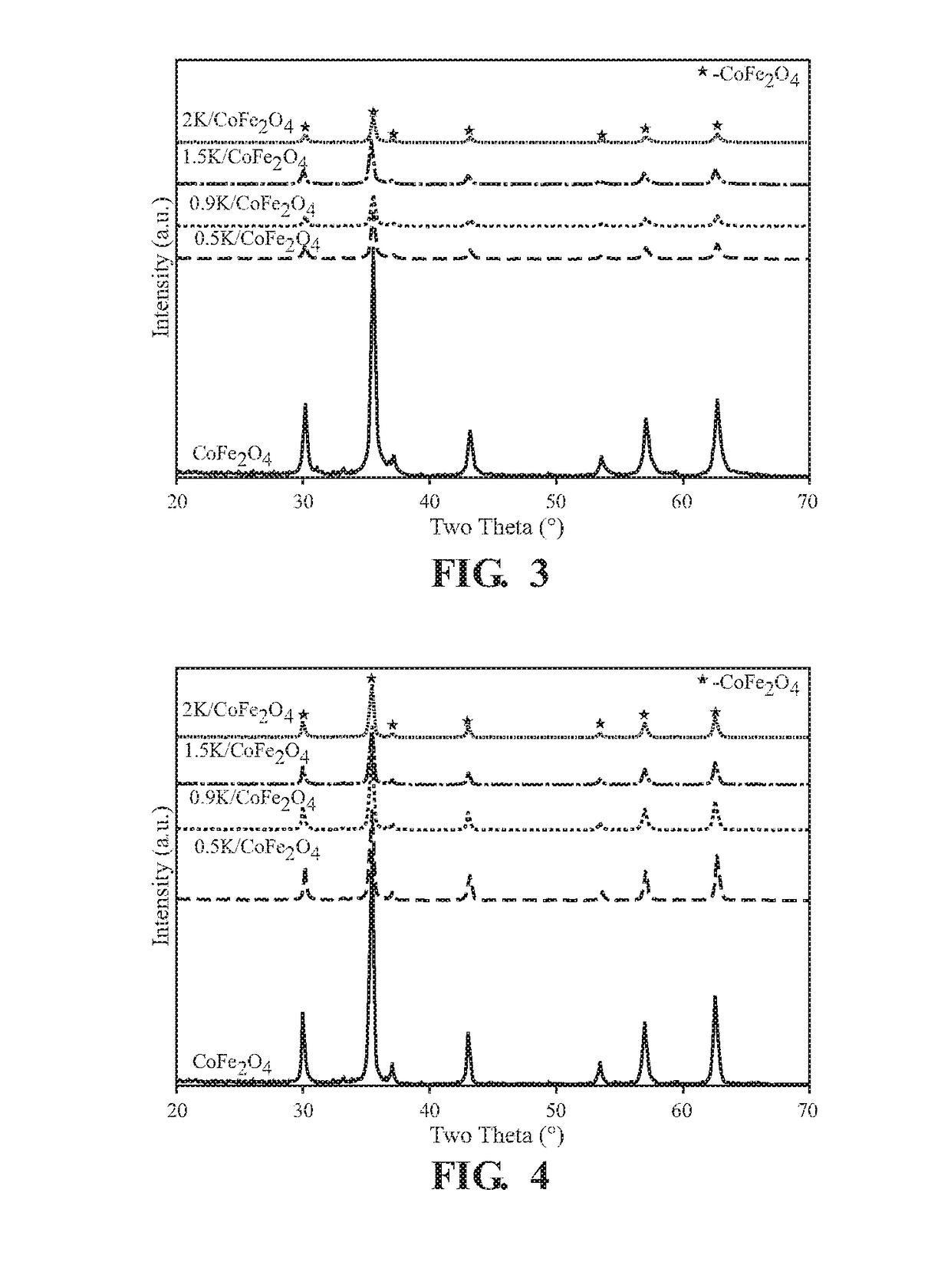
[0049]The phase composition of Examples 1-4 and the Comparative Example is measured using X-ray diffraction measurements. X-ray powder diffraction (XRD) measurements are performed using a Rigaku SmartLab X Ray Diffractometer. Spectra are collected over a 20 range of 20-80 degrees at a rate of 0.5 degrees per minute with a step size of 0.02 degrees per step. Structural assignments are made using PDXL software. The phase composition of the materials is determined using the ICDD-PDF database.
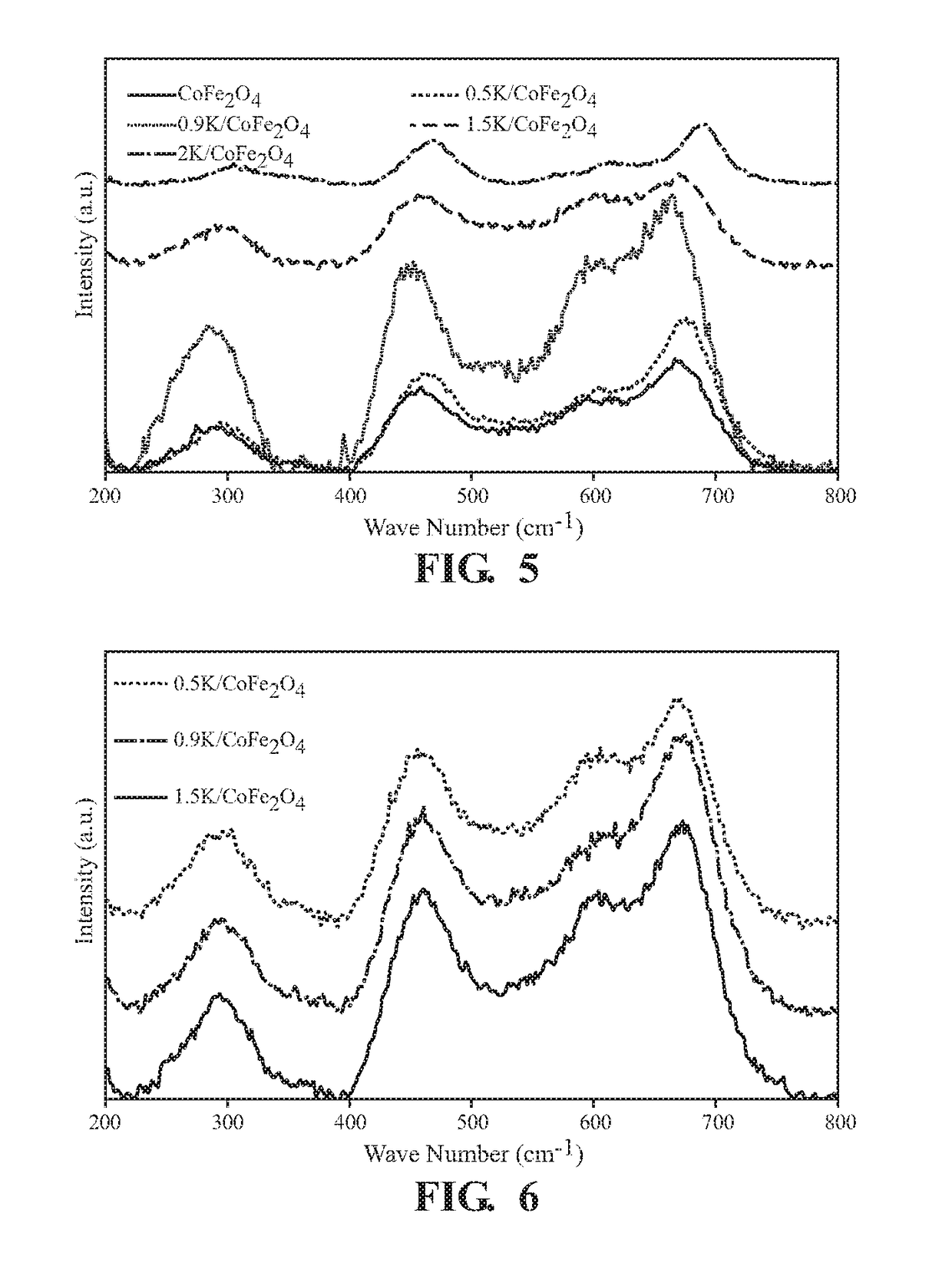
[0050]Raman spectra of the samples are recorded using a HORIBA Lab Ram HR 800 spectrometer with a 532 nm Ar+ ion laser. Laser power is optimized to 0.5 milliwatts on the sample surface, after taking into consideration the (S / N) ratio and sample degradation. Raman spectra are acquired for 60s using a 100× objective lens.
[0051]NO adsorption capacities of the CoFe2O4 and K / CoFe2O4 catalysts are measured using NETZSCH STA-449 thermogravimetric...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com