Methods for treating sleep disorders, sleep disturbances, and related symptoms using aminosterol compositions
a technology of aminosterols and sleep disorders, applied in the field of methods, can solve the problems of poor bioavailability, no anorectic activity, and inability to make therapeutic claims
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
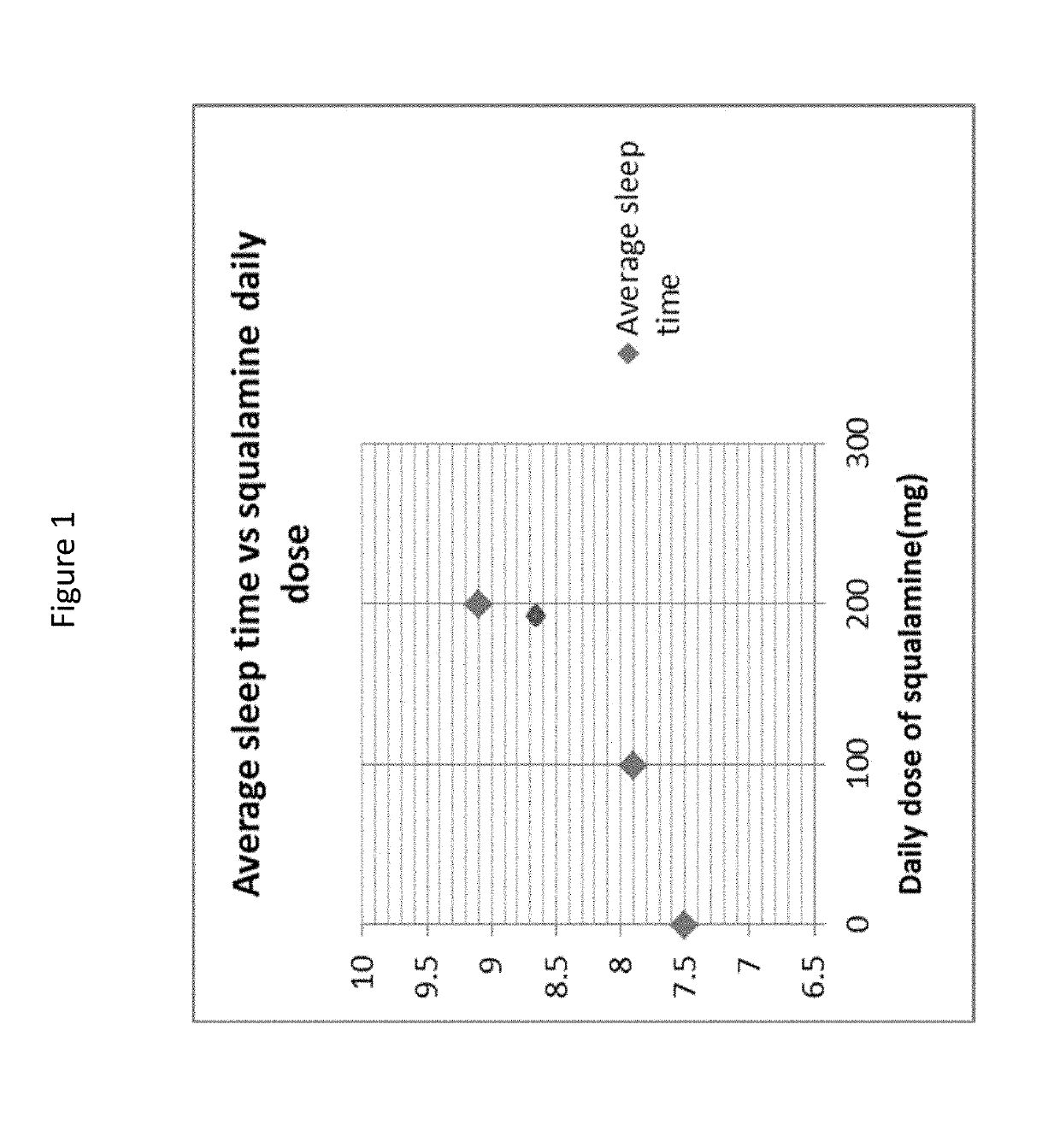
[0278]An elderly patient with a sleep disorder was treated with squalamine to determine the efficacy of the drug for treating sleep disturbances. The patient was a 78 year old woman with a 10 year history of sleep disturbance and a history of constipation, anxiety, and anosmia for as long as she could remember. Her sleep was profoundly disturbed: she had difficulty falling asleep, awoke 3-4 times during the night, had vivid dreams and nightmares, spoke and screamed out loud during her sleep (REM-behavior disorder, RBD), often saw “apparitions” or people in the room (e.g., a cook in a top hat, priests and angels dressed in white). She slept with a night light on in an attempt to minimize these occurrences.
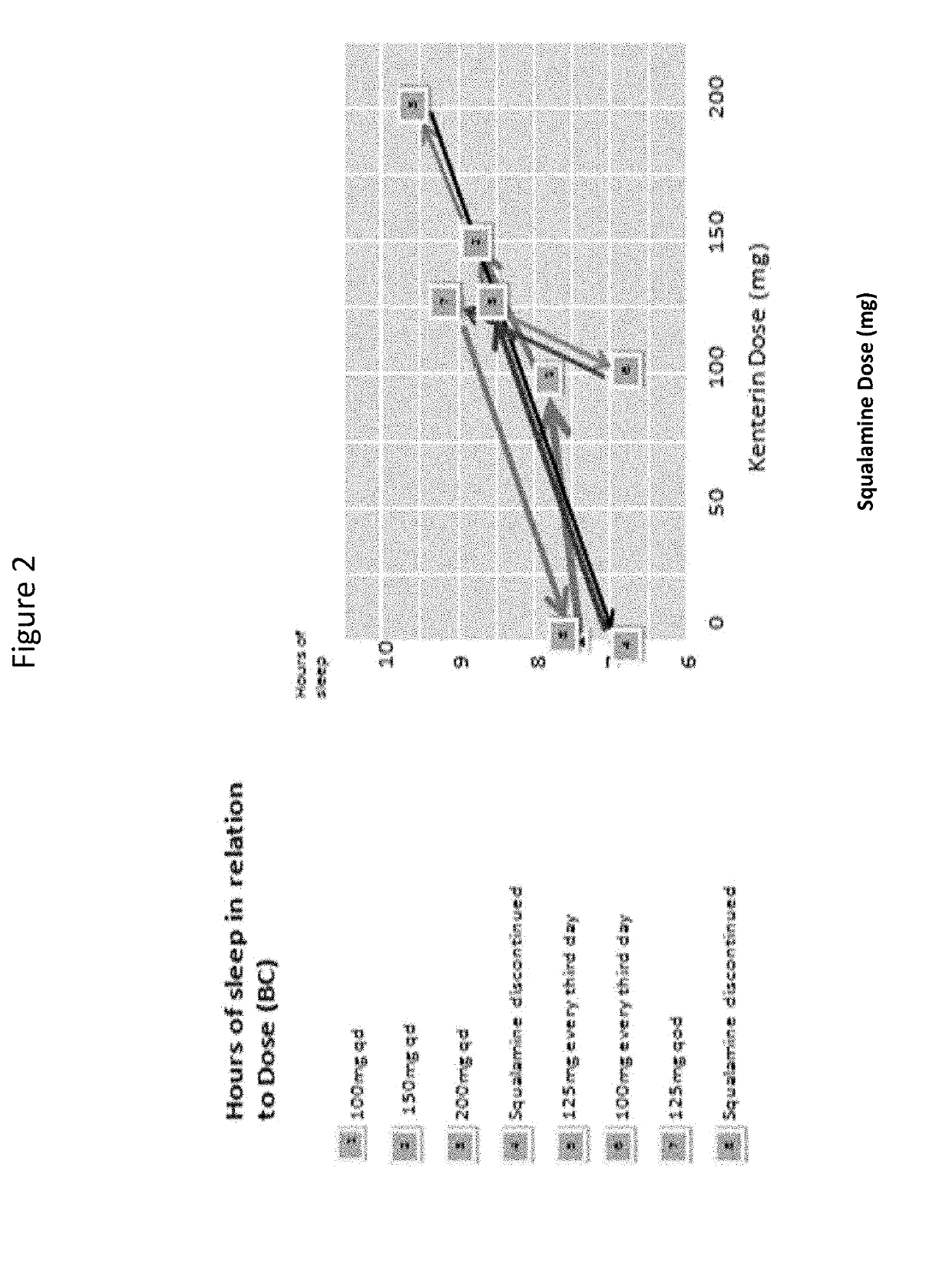
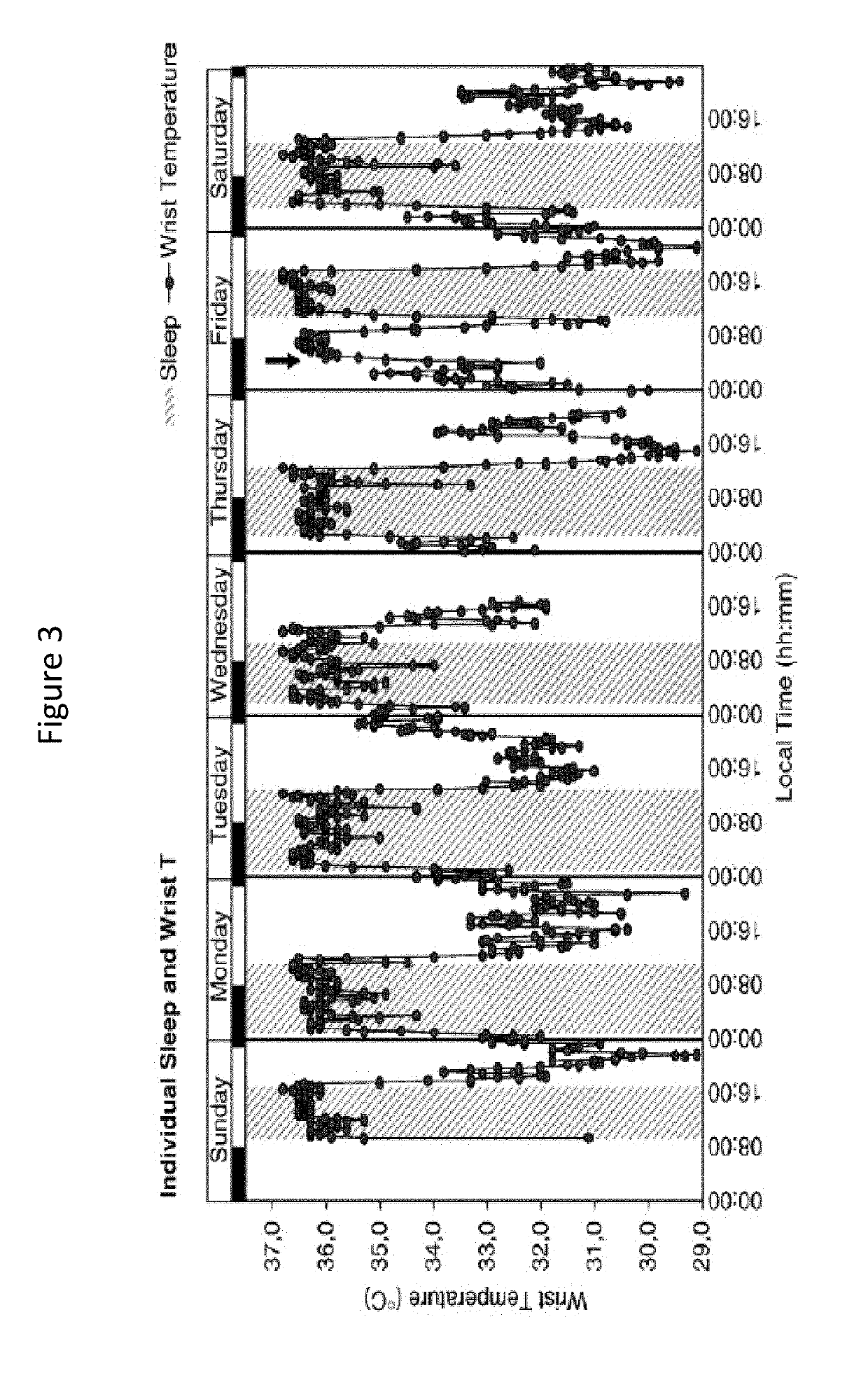
[0279]The patient was treated with oral squalamine (Kenterin™) starting at 100 mg qd and increasing to 200 mg qd. Sleep was monitored indirectly with the use of a temperature sensor (I-button) mounted on a wristband. The sensor was programed to record skin temperature at the wrist e...
example 2
[0289]An 82 year old patient had a 20 year history of sleeping disorder and life-long constipation. The patient had very significant delay in sleep onset, fragmented sleep, thrashing of arms and legs (RBD) and daytime somnolence. On polysomnography, he had apneic spells up to 17 seconds in duration with an oxygen desaturation as low as 93%. The patient slept no more than 3-4 hours, awakened 3-4 times, and each awakening lasted 30-60 minutes. Medications included clonazepam and laxatives.
[0290]Clonazepam and laxatives were discontinued and he was started on 100 mg of squalamine every other day at bedtime. This regimen had no effect on his sleep or constipation. The squalamine dose was then increased to 150 mg daily at bedtime, without effect. He was then switched to 150 mg daily taken in the morning. At that dose, his bowels regularized almost immediately, his arms and legs stopped thrashing in the night, his total sleep time increased, he started falling asleep much sooner, and he s...
example 3
[0299]Introduction
[0300]Squalamine has been administered orally to individuals with various medical conditions, including Parkinson's disease. A striking prokinetic effect on gastrointestinal (GI) motility as evidenced by relief of constipation, softening of stool consistency, and relief of GI pain and cramping (when present) has been observed in every treated individual. To better understand the mechanisms underlying the effect of squalamine a series of studies have been performed on both young and aged wild type mice and strains engineered as models of Parkinson's disease.
[0301]Results
[0302]Squalamine Normalizes Colonic Dysmotility Seen with Aging, Opiate Administration, and Parkinson's Disease
[0303]Isolated colons were mounted in an apparatus that permits continuous perfusion through the lumen, and the monitoring of the velocity, frequency and pressure of migrating muscular contractions (MMCs). Similar to the experience in humans, aging in mice is associated with diminished colon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| skin temperature | aaaaa | aaaaa |
| skin temperature | aaaaa | aaaaa |
| skin temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com