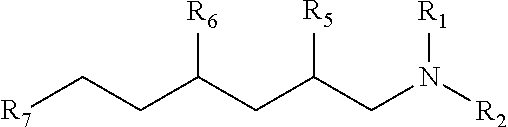
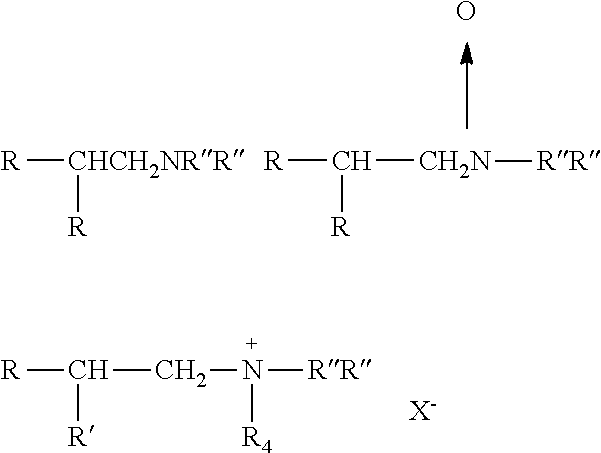
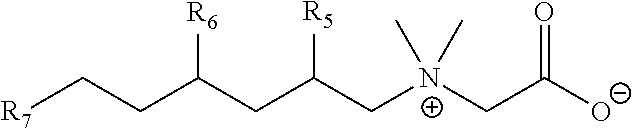
Branched trialkylamine precursors, intermediates, products made therefrom and processes of manufacture
a trialkylamine and precursor technology, applied in the field of organic chemistry, can solve the problems of increasing energy and facilities usage, affecting the performance of individual hydrophobes in the mixture, and not being known to perform as well as the mixtures, and achieves the effects of low foaming, effective oily soil and/or stain removal, and mild skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of C11 Enal (4-ethyl-2-methyloct-2-enal)
[0350]
[0351]To a solution of NaOH (250 g, 3.12 mol) in water (303 mL) was added tributylmethylammonium chloride (49 g, 0.156 mol). To this solution, a premix solution of 2-ethyl hexaldehyde (500 g, 3.90 mol) and propionaldehyde (190 g, 3.28 mol) was added dropwise over the period of 5 h. The addition was maintained such that the temperature of the reaction did not exceed 55° C. The contents of the reaction were stirred at 50° C. for 12 h. The reaction was cooled to room temperature and the contents were transferred to a separatory funnel. The aqueous layer was separated and the organic layer was washed sequentially with water (500 mL), saturated aq. NH4Cl (500 mL) and water (500 mL). The organic layer was separated, dried over MgSO4 and filtered. The pure enal of this Example was distilled at 85° C. (vapour temperature) under 4 mm reduced pressure.
example 2
of C11 enal 4-ethyl-2-methyloctanal (C11-aldehyde)
[0352]
[0353]The C11-enal obtained in Example 1 was hydrogenated using palladium (Pd)—C (1 mol %) under hydrogen pressure (400 psi) at 120° C. The resultant aldehyde in this Example 2, 4-ethyl-2-methyloctanal, was distilled at 80° C.-85° C. (vapor temperature) under 3 mm reduced pressure.
example 3
of 2,4-diethyl-2-octenal
[0354]Charge a 3 neck, 1 L (liter) flask with Water (133 ml), sodium hydroxide (56.7 g, 1418 mmol), and tetrabutylammonium chloride (25.0 g, 90 mmol). Using a magnetic stir bar, stir to dissolve. Attach a 500 mL liquid dropping funnel charged with butyraldehyde (75.1 g, 1042 mmol) and 2-ethylhexanal (257.2 g, 2006 mmol). Add the aldehyde mixture to the aqueous mixture in the pot dropwise over 2-3 h. After aldehyde addition is complete, pour the mixture into a 1 L separatory funnel. Separate the aqueous layer and wash the organic layer with brine (1×100 mL) and water (2×100 mL). Separate the organic layer, dry over MgSO4, and filter. The crude reaction mixture (190 g) contains 46% 2-ethylhexanal and 33% 2,4-diethyloct-2-enal. This corresponds to 100% conversion of n-butyraldehyde and 56.9% yield, based on moles of butyraldehyde, of 2,4-diethyloctenal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com