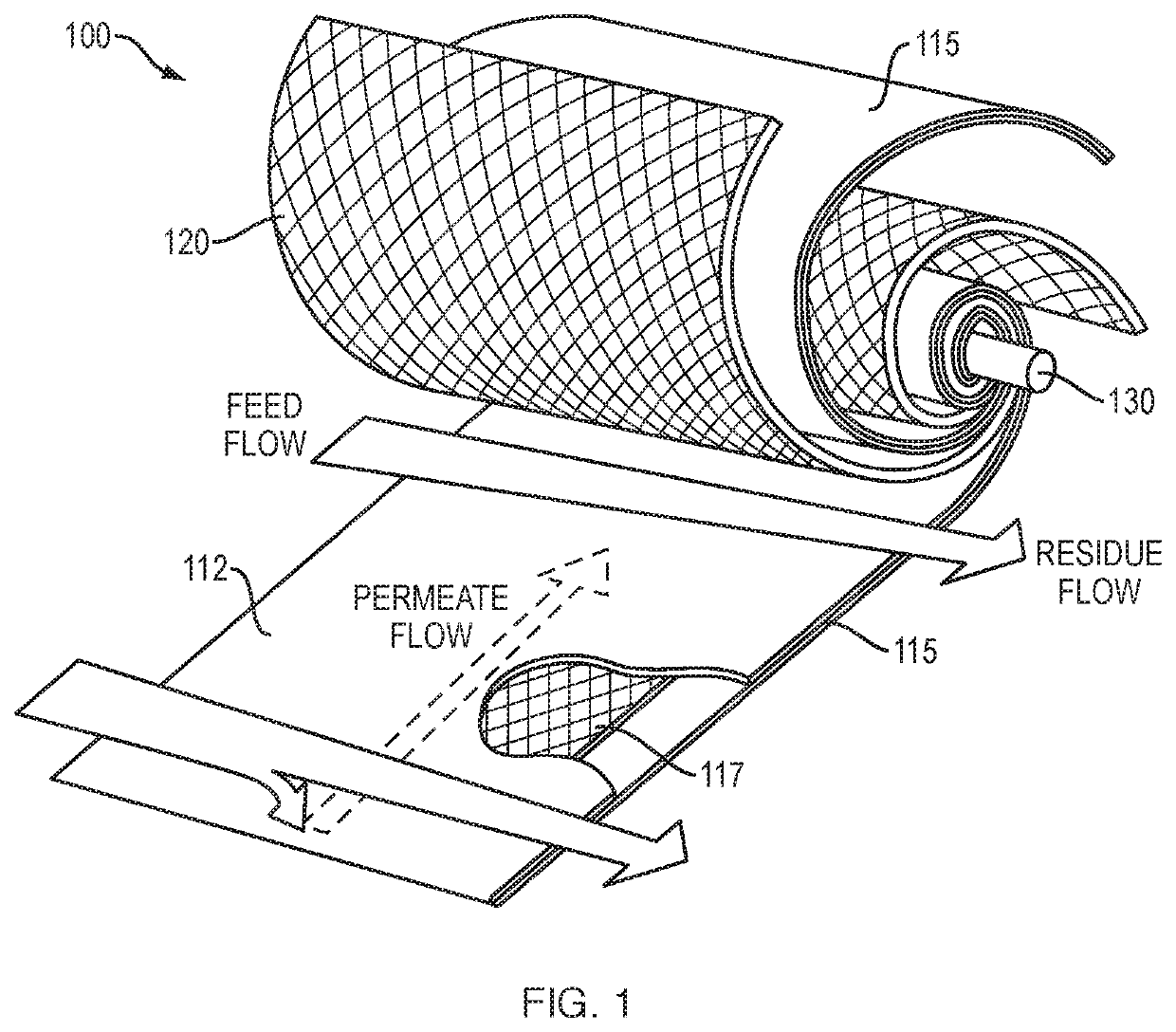
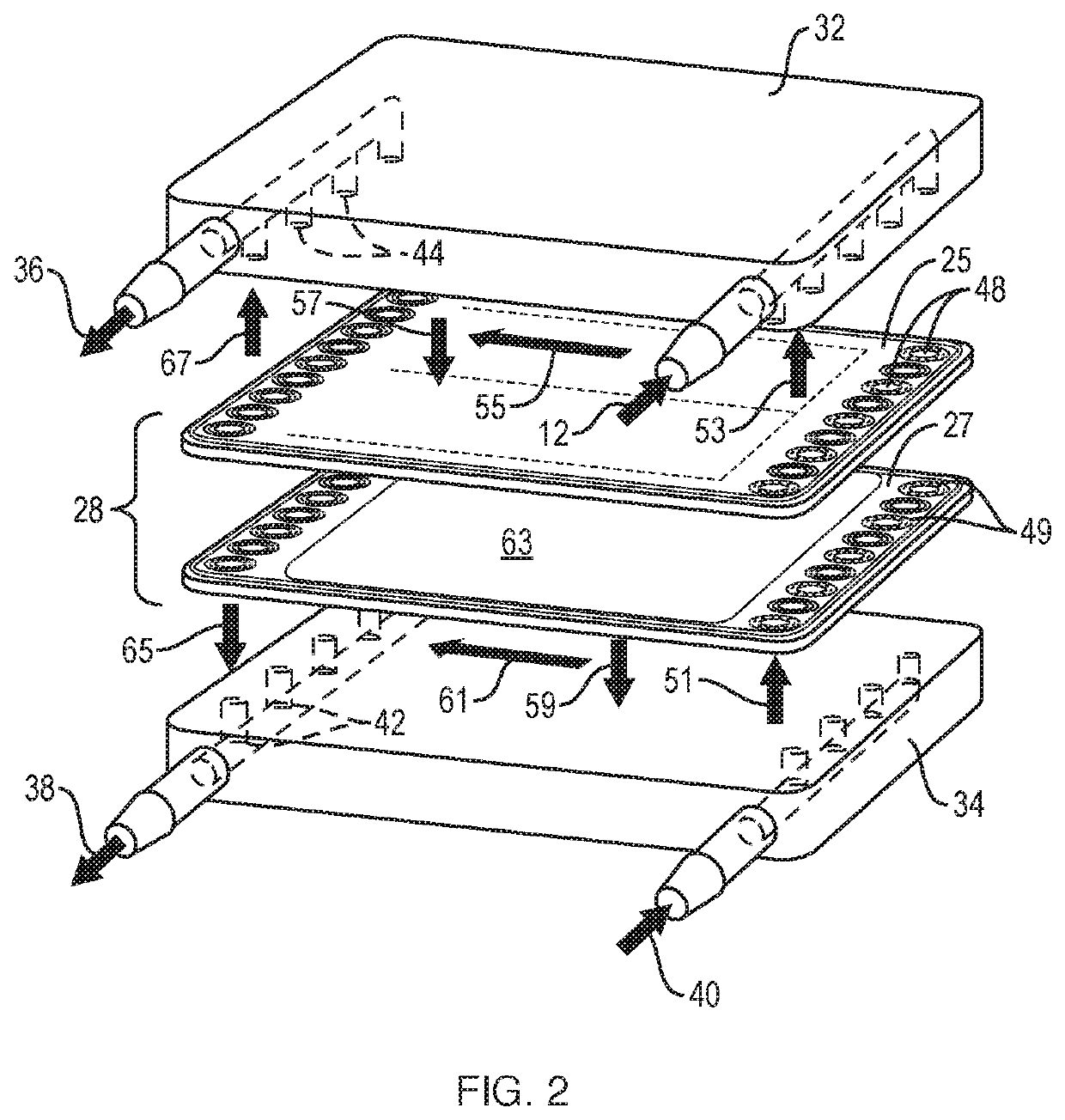
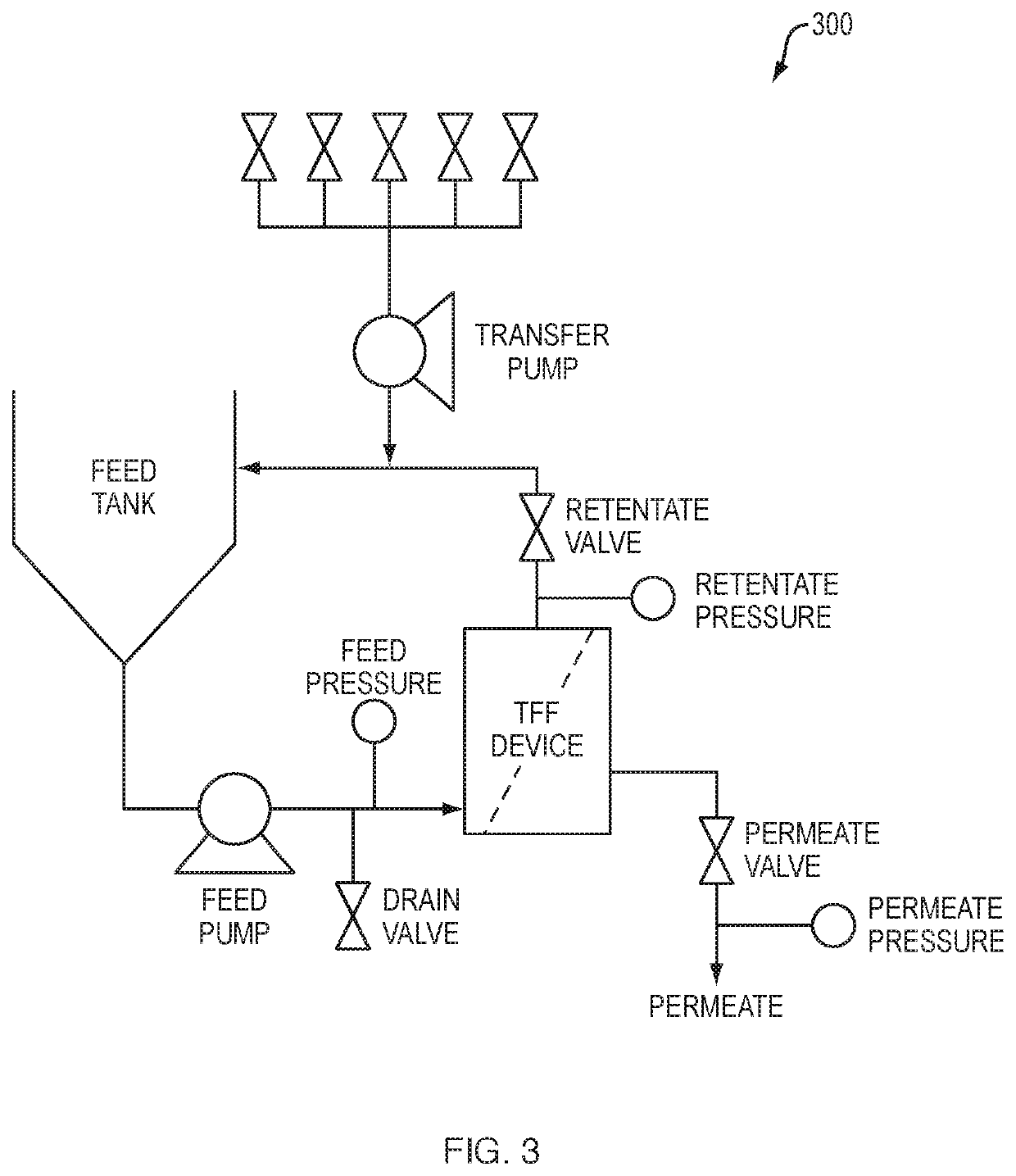
Tangential flow filtration device for perfusion applications
a filtration device and perfusion technology, applied in the field of tangential flow filtration devices for perfusion applications, can solve the problems of relatively short lifespan of filter elements used in such conventional systems and processes, and significantly reduced sieving at low harvest throughput, and achieve the effect of improving sieving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Feed Spacer Screens
[0088]A modeling study was carried out to evaluate the effects of feed screen parameters on shear rates. The geometry of each of three feed screens was generated for the model using the parametric geometry function in Autodesk Inventor (Autodesk, Inc. Boston, Mass.). The modeled feed screens included: (1) C-screens (Propyltex® screens, Sefar, QC, Canada) having a two-over-one twill pattern, an open area of 32%, a fiber density of 16.2 fibers / cm, fiber diameter of 270 μm, and a thickness of 515 μm; (2) D-screens (Propyltex® screens, Sefar, QC, Canada) having a two-over-one twill pattern, an open area of 36%, a fiber density of 12.2 fibers / cm, fiber diameter of 340 μm, and a thickness of 610 μm; and (3) D3-screens (Propyltex® screens, Sefar, QC, Canada) having a two-over-one twill pattern, an open area of 39%, a fiber density of 10.6 fibers / cm, fiber diameter of 360 μm, and a thickness of 645 μm. FIG. 4A illustrates a simulation of the D3 woven fiber feed spacer ...
example 2
n of Model Results with Experimental Results
[0091]Two prototype filter elements were created, each including a single filter element sheet arranged in Pellicon® 3 micro plate and frame format. Both filter element sheets included a 0.65 micron Durapore® membrane. One filter element sheet contained a D-screen feed spacer and the other contained a D3-screen feed spacer. The filter elements underwent testing in an AKTAcrossflow™ system (GE Healthcare Lifesciences, Marlborough, Mass.) with a cell culture solution containing CHO-S cells in a density of 30-60 million cells per milliliter in CHO Cellvento™ 110 media. Feed, retentate and permeate flow were controlled during the experiments and feed pressure measurements were obtained, as shown in FIG. 6. Obtained experimental feed pressures were compared with those of modeled devices using corresponding retentate and permeate pressure values. As shown in FIG. 6, the results of the modeled devices correlated well with feed pressure values as ...
example 3
f Filter Elements and Pumps
[0092]Six different cell retention systems (i.e., perfusion systems) were evaluated, including four systems having commercially available filter elements, and four systems having prototype filter elements. The systems included:
[0093](1) XCell™ ATF-2 system (Repligen, Waltham, Mass.) with diaphragm pump and a 0.13 m2, 0.2 micron PES hollow fiber filter element. The system was operated in alternating flow mode under recommended cross-flow rates.
[0094](2) XCell™ ATF-2 system with diaphragm pump and a 0.13 m2, 0.5 micron PES hollow fiber filter element. The system was operated in alternating flow mode under recommended cross-flow rates.
[0095](3) Magnetic levitation pump (Levitronix®) and 0.13 m2, 0.5 micron PES hollow fiber filter element. The system was operated in recirculation mode under recommended cross-flow rates.
[0096](4) Peristaltic pump (Watson Marlow) and Prostak™ cassette (MilliporeSigma, Billerica, Mass.) with 0.06 m2, 0.22 micron PVDF membrane. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com