Synthetic hybrid receptor and genetic circuit in bacteria to detect enteric pathogenic microorganisms
a technology of genetic circuit and hybrid receptor, applied in the field of biosynthetic engineering of microorganisms, can solve the problems of low cost, insufficient safety, efficient treatment, etc., and achieve the effects of reducing the spread of bacterial pathogens, rapid response, and saving patients' lives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ng of Lactococcus lactis to Detect and Kill Vibrio cholerae
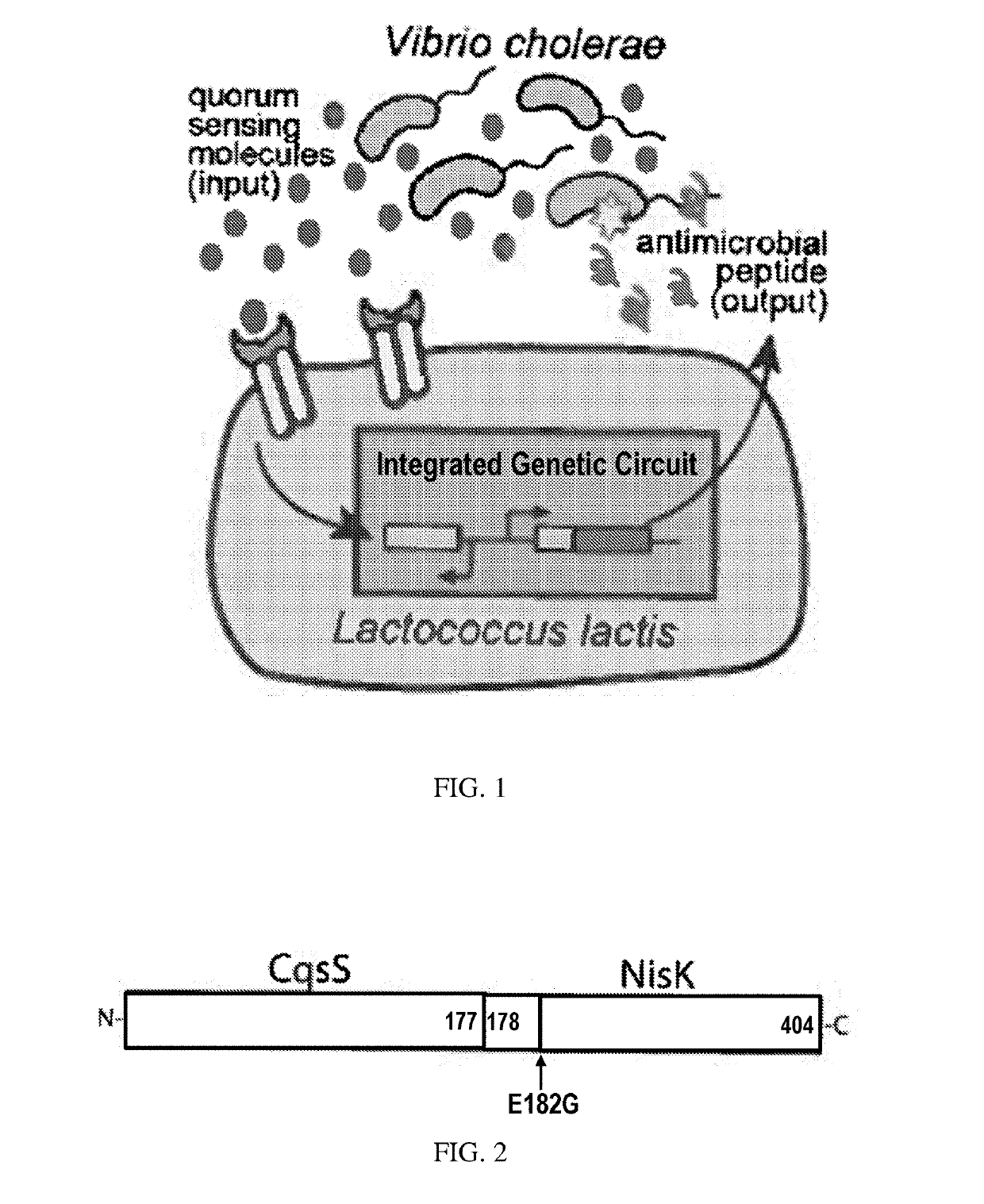
[0080]The purpose of this technology is to engineer the two-component system of the probiotic bacteria Lactococcus lactis to detect and kill the cholera pathogen Vibrio cholerae. L. lactis is a member of the lactic-acid bacteria family and labeled “generally recognized as safe (GRAS)” by the FDA. Because it can reside in the human intestine with no harm to the body, L. lactis can be an ideal candidate for intestinal pathogen sensing and killing. Cholera is an infectious disease that can cause severe diarrhea. There are three to five million cholera cases every year, resulting in I 00,000-120,000 deaths, mostly in developing countries[l]. The engineered L. lactis will promise a safe, efficient and low-cost treatment for the serious infectious disease cholera.
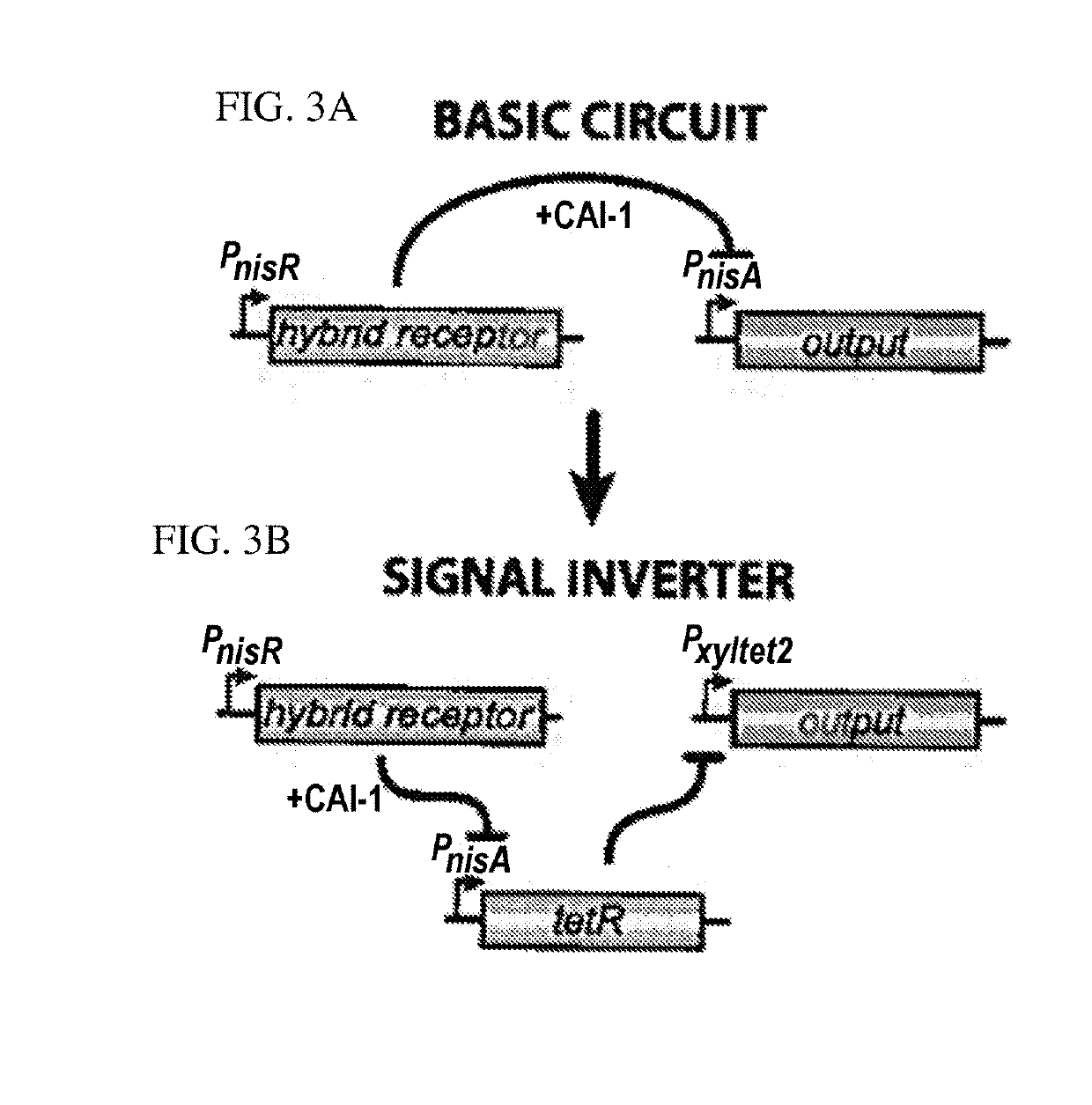
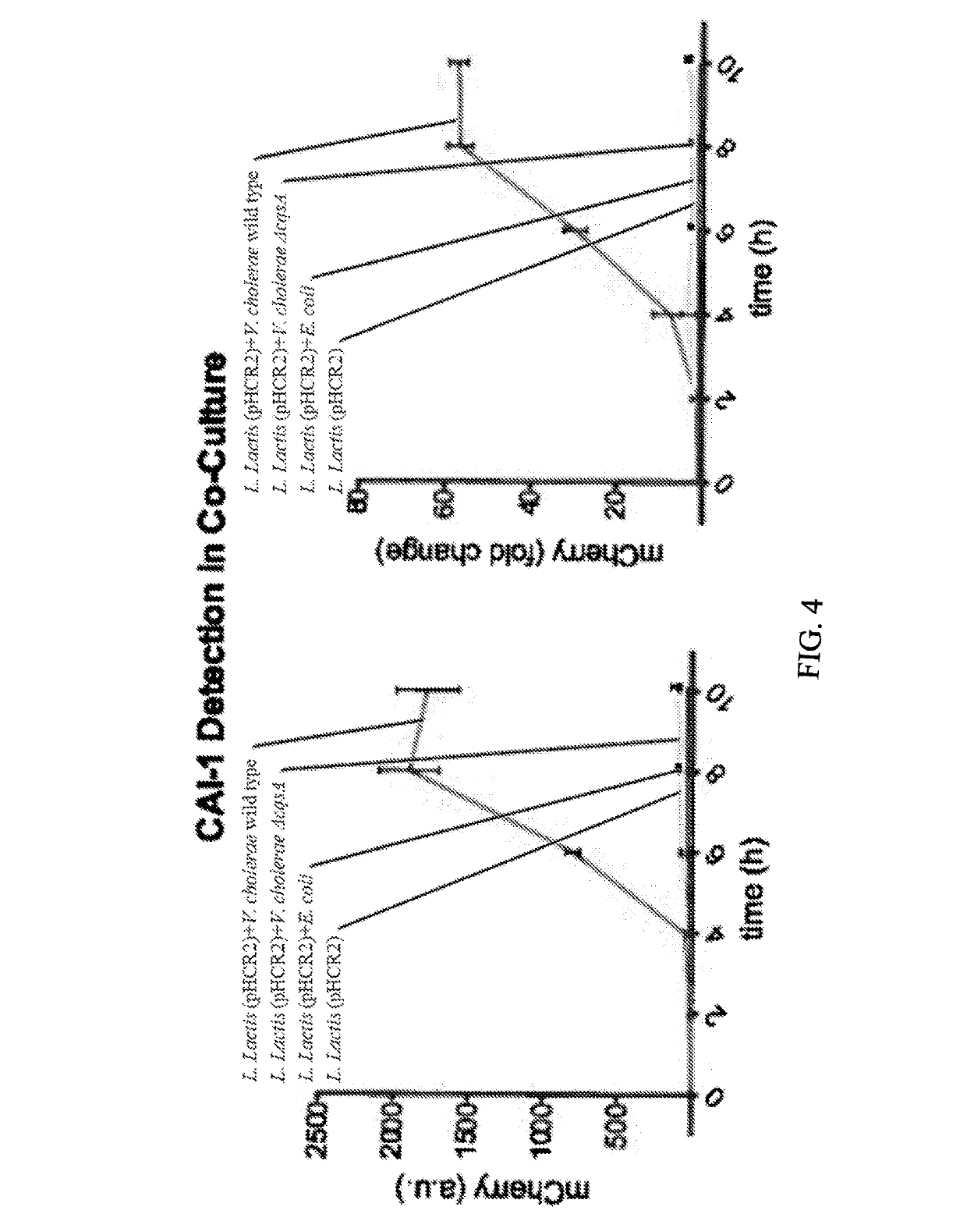
[0081]A hybrid cell surface receptor that allows L. lactis to detect the Vibrio cholerae quorum-sensing molecule CAI-1 has been developed[2]. To enable CAI-1 detection, ...
example 2
ng of Lactococcus lactis to Detect Vibrio cholerae Using a Colorimetric Based Assay
[0092]The present example describes an engineered Lactococcus lactis (L. lactis) microorganism that can be used to detect (e.g., in vitro) a V. choerae microorganism in a colorimetric assay. L. lactis was engineered to produce ß-lactamase in response to detecting CAI-1 secreted by V. cholerae, which turns a yellow substrate (nitrocefin) red when V. cholerae is detected. β-lactamase is a robust enzyme with a high catalytic efficacy and a small size that makes it easy to diffuse. Wild-type (w.t.) L. lactis were transformed with pHTR plasmid, a schematic of which is shown in FIG. 9. The pHTR plasmid is a derivation of the pHCR2 plasmid, with the mCherry gene replaced by β-lactamase gene. L. lactis transformed with pHTR express ß-lactamase in response to detecting CAI-1 secreted by V. cholerae. The pHTR construct has β-lactamase repressed by TetR, where anhydrotetracycline (ATc) is an inducer that can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| colorimetric | aaaaa | aaaaa |
| color change | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com