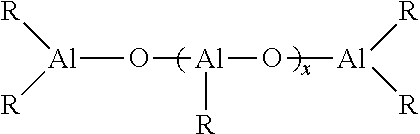
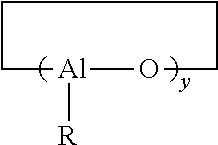
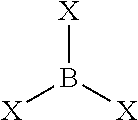
Aged lanthanide-based catalyst systems and their use in the preparation of cis-1,4-polydienes
a technology of lanthanide-based catalysts and cis-1,4-polydienes, which is applied in the field of preformed lanthanide-based catalyst systems and their use in the synthesis of cis1, 4polydienes, can solve the problems of high catalyst loading of lanthanide catalysts, high cost of lanthanide compounds, and high cost of cis-1,4-polydienes prepared with lanthanide-based catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i (
Samples 1-8)
[0138]In order to demonstrate the beneficial properties observed when aged catalyst compositions are employed to prepare cis-1,4-polydienes, the following catalyst compositions were prepared and employed to synthesize polydienes.
[0139]Unless otherwise specified, the Mooney viscosities (ML1+4) of the polymer samples were determined at 100° C. by using a Monsanto Mooney viscometer with a large rotor, a one-minute warm-up time, and a four-minute running time. The number average (Mn) and weight average (M) molecular weights of the polymer samples were determined by gel permeation chromatography (GPC). The GPC instrument was equipped with a differential refractive index (RI) detector and an ultraviolet (UV) absorption detector. The cis-1,4-linkage, trans-1,4-linkage, and 1,2-linkage contents of the polymer samples were determined by infrared spectroscopy. The GPC UV / RI ratio, which is the ratio of the UV detector signal to the RI detector signal, was used to calculate the % f...
example ii (
Samples 8-14)
[0150]In order to demonstrate the beneficial properties observed when aged catalyst compositions are treated with additional alkylating agent, the following catalyst compositions were prepared and employed to synthesize polydienes.
[0151]A preformed catalyst solution was prepared by sequentially mixing 11.76 mL of a 20.1 wt. % solution of butadiene in hexanes, 2.69 mL of a 0.54 M neodymium versatate solution in cyclohexanes, and 13.38 mL of a 1.08 M diisobutylaluminum hydride solution in hexanes. The mixture was allowed to stand at room temperature for 3 minutes and then 2.17 mL of a 1.00 M ethylaluminum dichloride solution in hexanes was added. After an additional 10 minutes of aging at room temperature the catalyst mixture was diluted with 4.00 mL of hexanes.
Sample 8
[0152]A nitrogen purged sealed glass vessel was charged with 84.6 g of anhydrous hexanes and 248.8 g of a 20.1 wt. % solution of butadiene in hexanes. To this solution was added 2.00 mL of the preformed cat...
example iii (
Samples 15-20)
[0160]In order to demonstrate the beneficial properties observed when aged catalyst compositions are treated with additional alkylating agent, the following catalyst compositions were prepared and employed to synthesize polydienes.
CatalystAA (No Catalyst Aging):
[0161]A catalyst solution was prepared by sequentially mixing 8.50 mL of a 0.11 M neodymium versatate solution in cyclohexanes, 10.79 mL of a 21.3 wt. % solution of butadiene in hexanes, 10.89 mL of a 1.03 M diisobutylaluminum hydride solution in hexanes, 2.46 mL of a 0.43 M ethylaluminum sesquichloride solution in hexanes, and 4.75 mL of hexanes. After addition of each individual chemical component to the catalyst mixture, the mixture was allowed to stand for 10 minutes.
Catalyst BB (Aged 7 Days):
[0162]A catalyst solution was prepared by sequentially mixing 8.50 mL of a 0.11 M neodymium versatate solution in cyclohexanes, 10.79 mL of a 21.3 wt. % solution of butadiene in hexanes, 10.89 mL of a 1.03 M diisobutyla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com