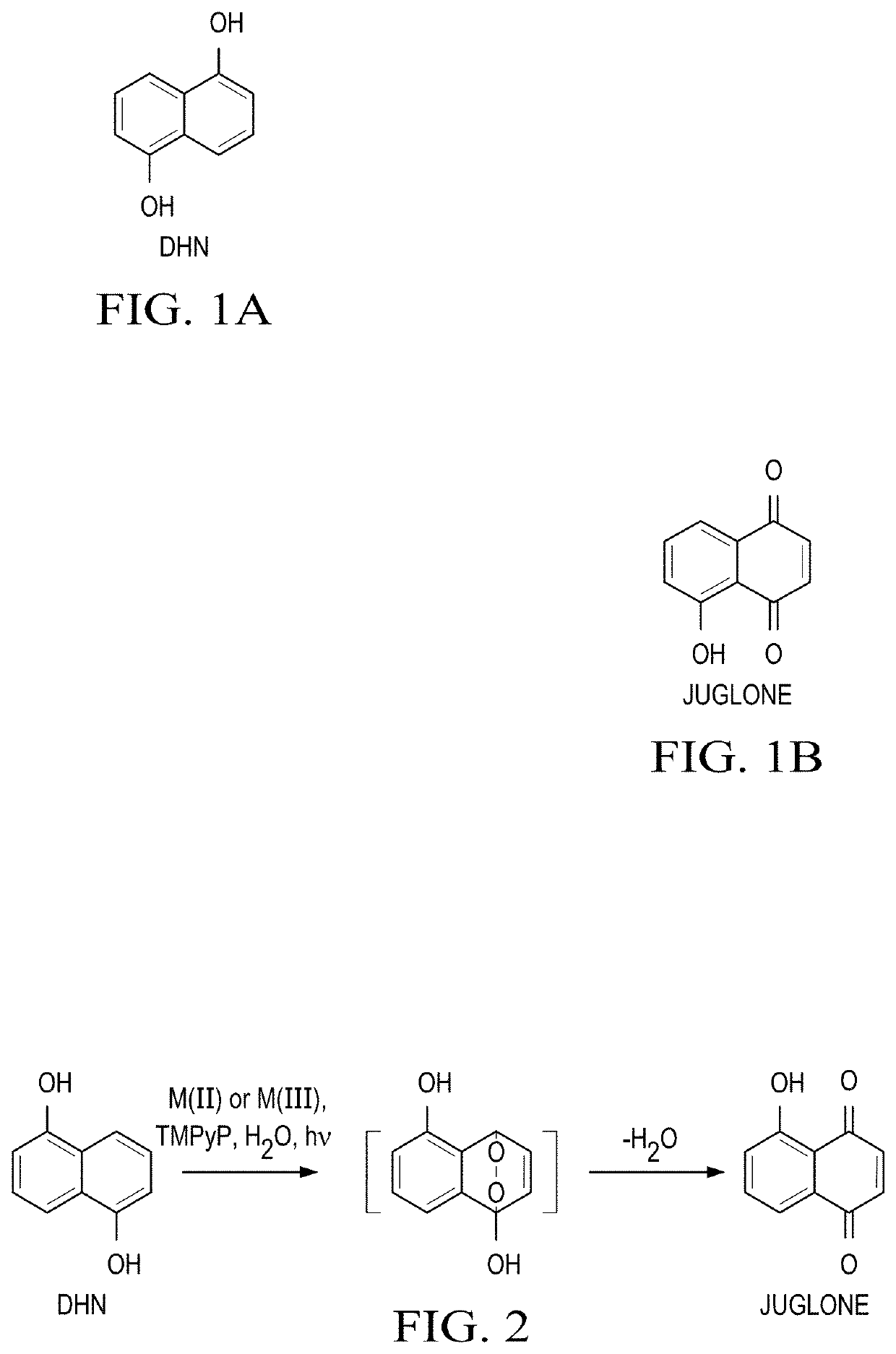
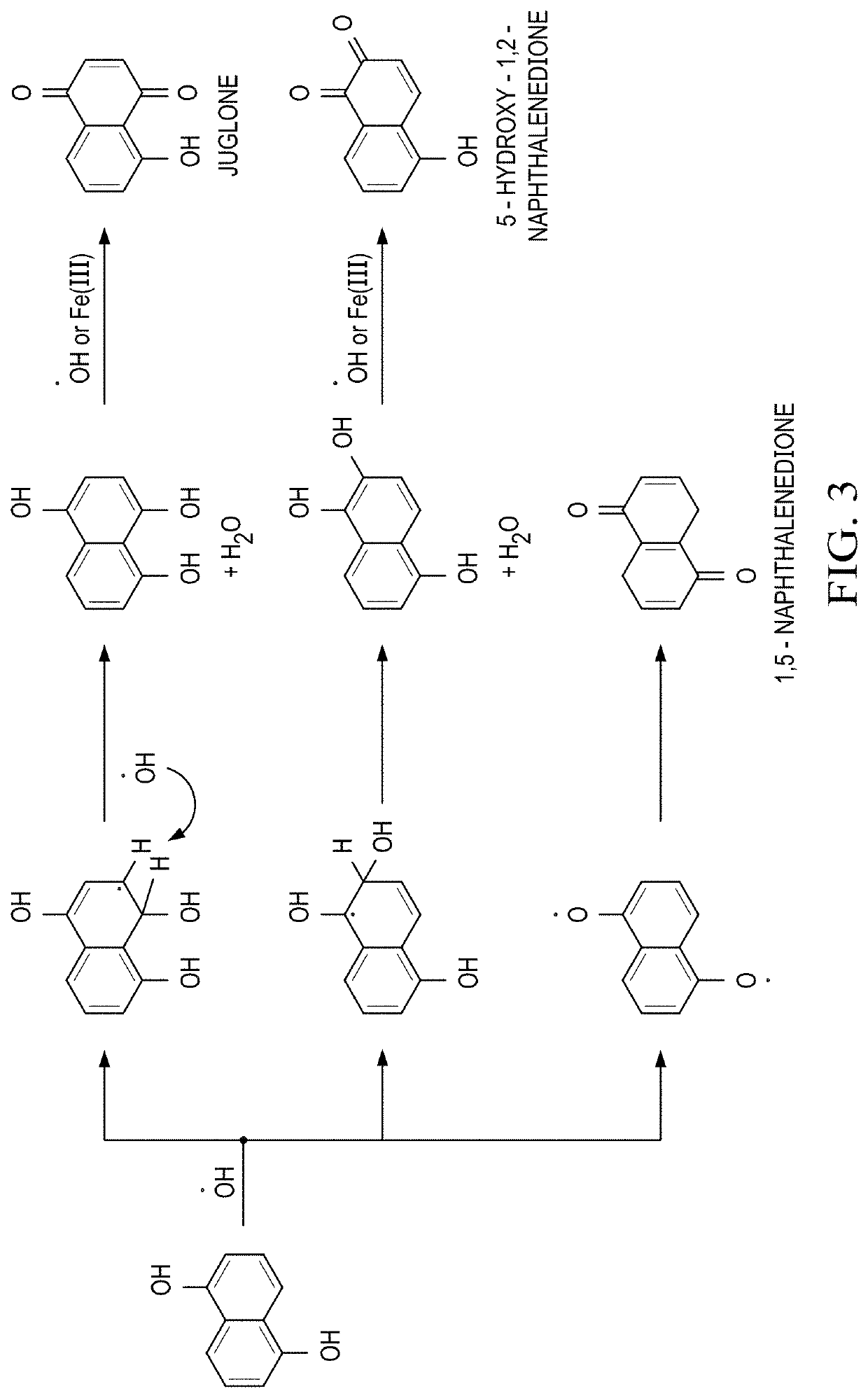
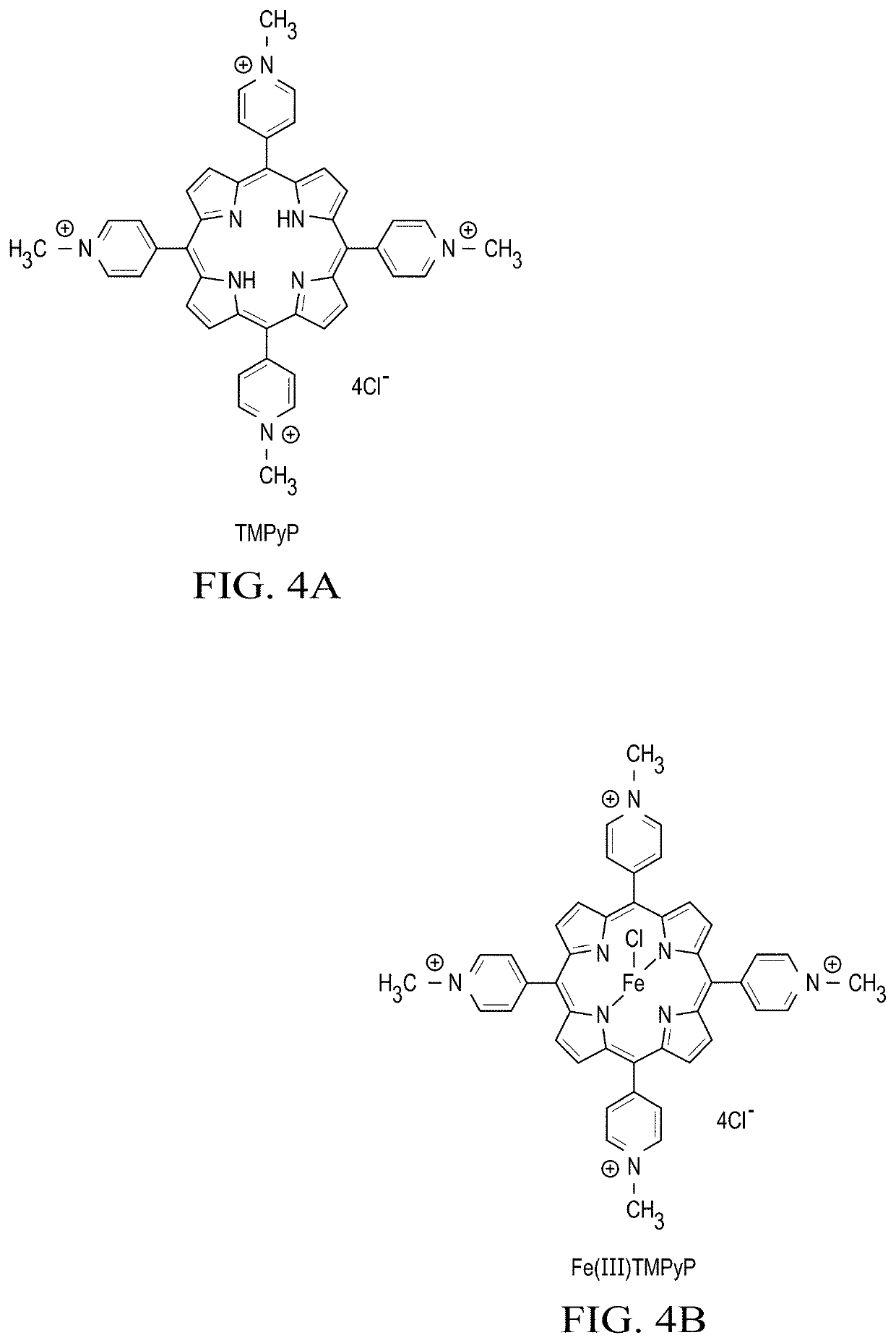
Multifunctional Treatment And Diagnostic Compositions And Methods
a diagnostic composition and multi-functional technology, applied in the field of multi-functional treatment and diagnostic compositions and methods, can solve the problems of lack of selectivity for removing or killing malignant tumor tissues, high cost or high invasiveness, and inability to administer toxic treatments, etc., and achieves the effect of reducing cost and being easy to prepar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0120]First, an experiment was performed in order to detect the generation of singlet oxygen (1O2) from TMPyP under visible light irradiation at 532 nm by using singlet oxygen sensor green (SOSG) in aqueous solution of TMPyP.
[0121]FIG. 7 shows in embedded window the fluorescence intensity of SOSG at 525 nm gradually increased with increasing amount of irradiation time indicating the generation of 1O2 in aqueous solution. The fluorescence spectra of the SOSG emission intensity was recorded immediately after irradiation. As shown in FIG. 7 embedded window, the emission intensity increased significantly after 60 min of irradiation with 532 nm light. Other experiments indicate that the fluorescence emission intensity of SOSG in aqueous TMPyP solution greatly increased in D2O solvent compared to H2O and substantially decreased in presence of NaN3. This data indicates that the aqueous solution of TMPyP generates 1O2 upon irradiation with 532 nm light showing that TMPyP is useful as a sing...
example 2
[0127]The effect of Fe(III) ions on photooxidation of DHN was investigated. Iron metal is an essential nutrient to the human body and it helps to operate many crucial functions including cell replication, metabolism, and growth in the mammalian cells. On the other hand, iron is a transition metal which has the capability to accept or lose electrons and take part in the free radical formation reactions.
[0128]FIG. 9 is a plot of the rate of change over 10 minutes time of DHN monitored at 301 nm as a function of irradiation time in aerobic conditions. Experiments were conducted in the presence of DHN (1.2×10−4 M) and TMPyP (6.0×10−6 M), and (i) Iron (III) (1.5×10−4 M) (square); (ii) without Iron (III) (triangle); (iii) Iron (III) (3.0×10−5 M) (cross); Iron (III) (5.0×10−5 M) (diamond); and Iron (III) (1.0×10−4 M) (circle).
[0129]As shown in FIG. 9, the results of investigation of the effect of Fe(III) ions on photooxidation of DHN demonstrates that the rate of photooxidation of DHN by T...
example 3
[0132]To find the nature of produced ROS in the treatment composition (DHN / TMPyP / Fe(III) ions) solution, a series of control reactions were carried out using above described materials, solutions, apparatus and methods.
[0133]Refer to FIG. 10, which is a plot of the rate of change over 20 minutes of DHN monitored at 301 nm as a function of irradiation time. Experiments were conducted in the presence of DHN (1.2×10−4 M), TMPyP (6.0×10−6 M) and NaN3 (100 mM) (plotted as crosses); DHN (1.2×10−4 M), TMPyP (6.0×10−6 M) and D2O (plotted as circles); DHN (1.2×10−4 M), TMPyP (6.0×10−6 M), and Iron (1.0×10−4 M) (plotted as squares); and DHN (1.2×10−4 M), TMPyP (6.0×10−6 M), and H2O (plotted as triangles).
[0134]The rate of DHN photooxidation by TMPyP / Fe(III) ions was found to increase dramatically in D2O compared to in H2O indicating the presence of singlet oxygen (1O2), as shown in FIG. 10. Also, significantly slower photooxidation of DHN by TMPyP / Fe(III) ions was observed in the presence of N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole ratios | aaaaa | aaaaa |
| mole ratios | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com