Method for preparing monosilane using trialkoxysilane
a monosilane and trialkoxysilane technology, applied in the field of monosilane preparation, can solve the problems of difficult to widely use the method of monosilane preparation, need to remove a starting reagent, increase in the price of a final product, etc., and achieve the effect of improving the preparation process, improving the performance and facilitating the removal of starting reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
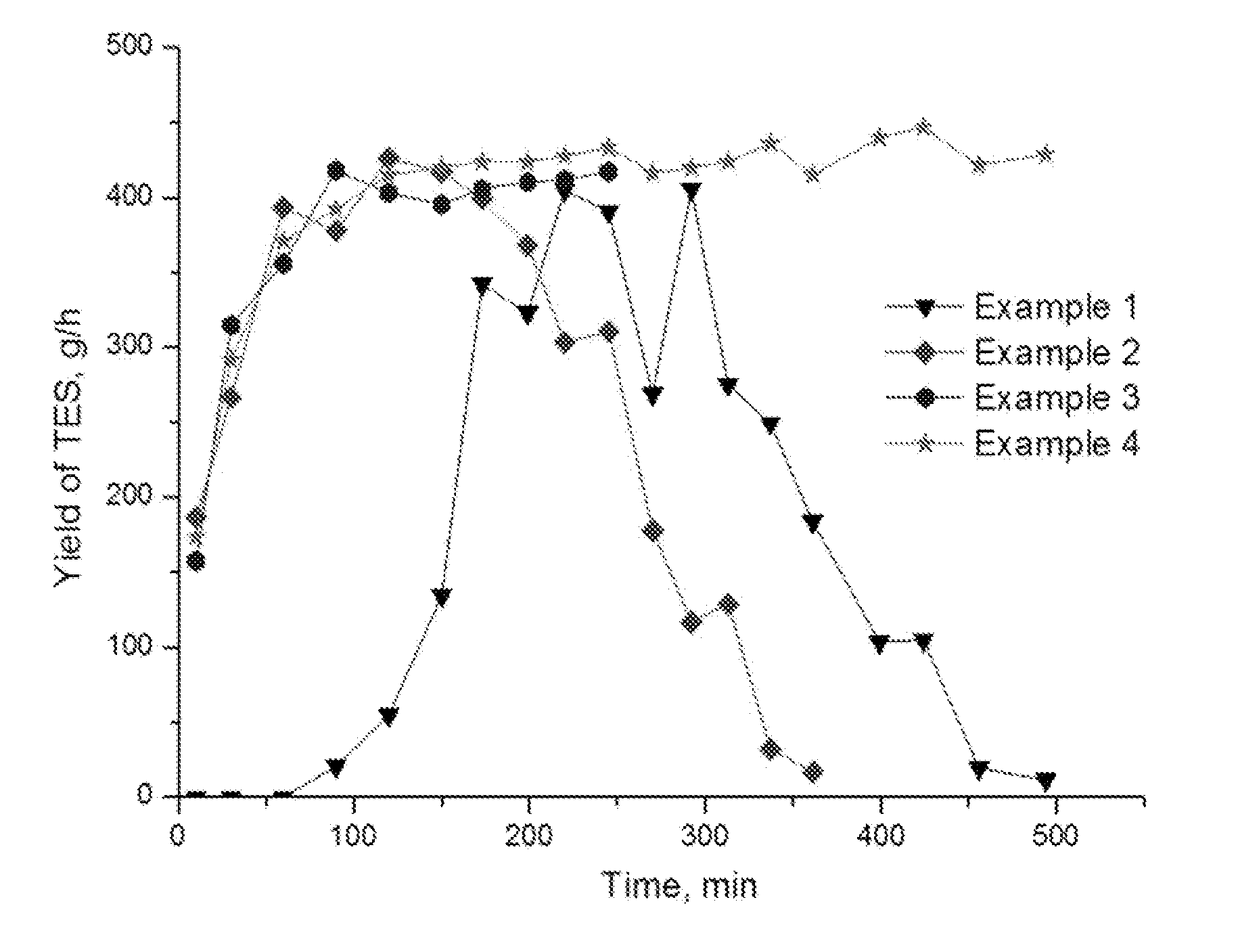
[0106]Silicon metal was pulverized in air using a planetary mill until the particle size thereof became 30 μm to 100 μm. 3.3 kg of pulverized silicon, 6.6 kg of a solvent, THERMINOL® 66, and 0.2 kg of the catalyst CuCl were put into a reactor. The contact material was heated to 242+2° C. while a stirrer is continuously operated at a rate of 850 rpm, and dried alcohol (ethanol) began to be supplied to the reactor at a maximum rate of 600 mL / h using a metering pump (digital dosing pump) GRUNDFOS® DME 60-10 AR. From the instant when a liquid product was produced from the reactor, the sample was collected every 30 minutes. When observing a sample analysis using gas chromatography Agilent® GC7890A, the synthesis reaction of triethoxysilane taking place as a result of reaction of silicon metal with ethyl alcohol began to take place 150 minutes (initial induction period) after alcohol was supplied thereto, and the intensity of the reaction was gradually increased (see the curve of Example ...
example 2
[0107]An experiment was conducted in the same manner as in Example 1, but the preparation environment of the reaction reagent was completely different. According to the proposed method, 3.3 kg of silicon metal was continuously pulverized in 6.6 kg of solvent, THERMINOL® 66. In the pulverization process, 0.2 kg of copper (I) chloride was introduced into a liquid suspension. Alcohol was supplied to the reactor, and then the synthesis reaction began after the initial reaction induction period of 10 minutes, the reaction rate increasing thereafter for 60 minutes (see the curve of Example 2 in FIG. 1). After 180 minutes, the synthesis reaction rate of triethoxysilane began to decrease, and the reaction was completely terminated 260 minutes after supplying alcohol. 1635 g of triethoxysilane and 105 g of tetraethoxysilane were obtained. The selectivity of triethoxysilane reached 94%.
example 3
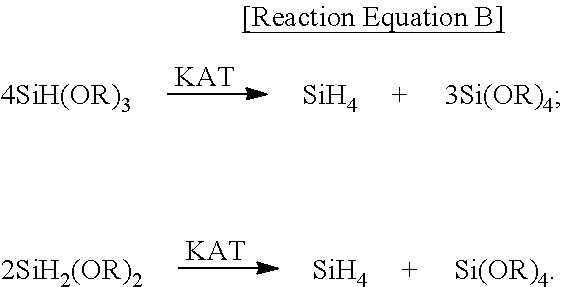
[0108]An experiment was conducted in the same manner as in Example 2, but there is a significant difference in that a continuous process was performed by continuously supplying a liquid suspension of silicon and solvent according to the proposed method to a reactor, and the ratio of mass consumed during the reaction with ethyl alcohol was 1:2. That is, during the synthesis process of alkoxysilane, a liquid suspension of silicon was supplied thereto at a rate suitable for the consumption rate of silicon according to the reaction. The amount of silicon consumed per unit time was calculated according to the mass balance equation of the following Equation 1.
mSi=k1·mTES+k2·mTEOS [Equation 1]
[0109]wherein mSi is a mass of silicon consumed as a result of direct reaction for unit time, mTES is a mass of triethoxysilane prepared as a result of direct reaction for unit time, mTEOS is a mass of tetraethoxysilane prepared as a result of direct reaction for unit time, coefficient k1 is a molecu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com