Process for the preparation of taxanes from 10-deacetylbaccatin III
a technology of baccatin and taxanes, which is applied in the field of process for the preparation of taxanes from 10deacetylbaccatin iii, can solve the problems of whose industrial preparation is particularly complex, and achieve the effect of higher yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
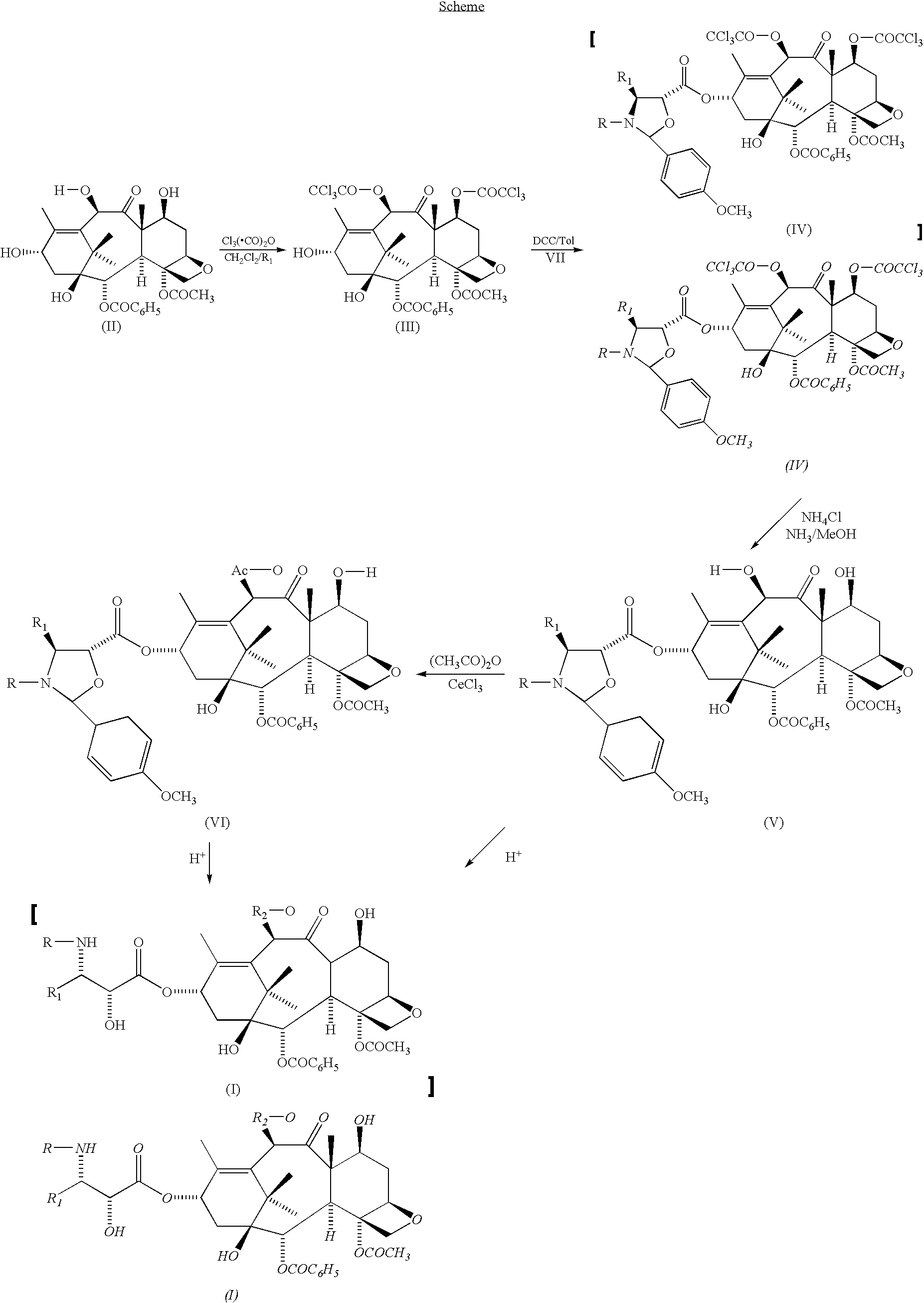
Preparation of 7,10-bis-trichloroacetyl-10-deacetylbaccatin III
[0034]A solution of 10 g of 10-deacetylbaccatin III (18.4 mmol) in 125 ml of dry methylene chloride and 42 ml of pyridine is added dropwise with 4.77 ml of trichloroacetic anhydride (42.32 mmol). The reaction mixture is stirred for three hours or anyhow until completion of the reaction, checked by TLC on silica gel using a 5:5 n-hexane / ethyl acetate mixture as eluent. Upon completion of the reaction, 5 ml of methanol are added to destroy the trichloroacetic anhydride excess, then water. The organic phase is thoroughly washed with HCl (0.1 M solution in water) to remove pyridine, whereas the remaining organic phase is dried over MgSO4 and concentrated to dryness under vacuum. A pale yellow solid (17 g) is obtained, which upon crystallization from chloroform shows the following chemical and spectroscopical characteristics:
[0035]IR (KBr) 3517, 1771, 1728, 1240, 981, 819, 787, 675 cm−1;
[0036]1H-NMR (200 MHz); δ8.11 (Bz AA′),...
example 2
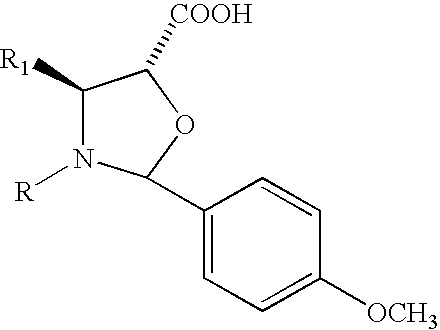
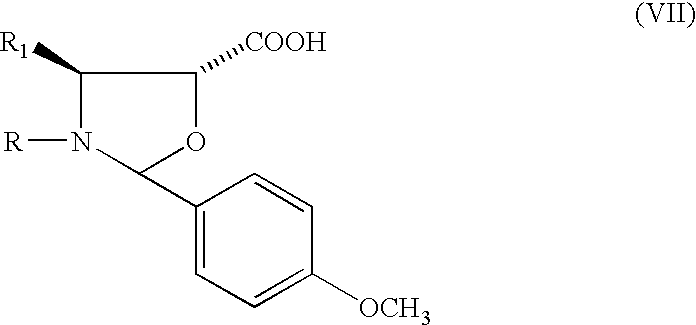
Preparation of 13-(2-(4-methoxyphenyl)-N-benzoyl-4-phenyl-oxazolidyl-)-10-deacetylbaccatin III
[0037]17 g of 7,10-bistrichloroacetyl-10-deacetylbaccatin III are dissolved in 250 ml of anhydrous toluene and added under stirring with 12.6 g of 2-(4-methoxyphenyl)-N-benzoyl-4-phenyl-oxazolidine-5-carboxylic acid and 6 g of DCC. After stirring overnight at 40° C., the reaction mixture is filtered and concentrated to dryness. The residue is dissolved in 300 ml of methanol / tetrahydrofuran and added with 24 ml of a 2M NH3 aqueous solution. After 1.5 hours at room temperature the reaction mixture is concentrated to small volume under vacuum, then diluted with water and the whole is extracted with ethyl acetate. The extract is concentrated to dryness and the residue is purified on a silica gel column, eluting the product with a 1:1 ethyl acetate / petroleum ether mixture, to obtain 16.8 g of the title product with m.p. 135° C. and [α]D=−58° (MeOH, C=0.5).
example 3
Preparation of 13-(2-(4-methoxyphenyl)-N-benzoyl-4-phenyl-oxazolidyl)-baccatin III
[0038]A solution of 13.7 g of the product of example II in 200 ml of tetrahydrofuran is added with 56 ml of a 10% suspension of CeCl3.7H2O in tetrahydrofuran, followed by 5.5 ml of acetic anhydride. After stirring overnight at room temperature, the reaction mixture is filtered, the filtrate is treated with methanol and concentrated to small volume; the mixture is diluted with H2O and the product is extracted with ethyl acetate, to obtain 12 g (84%) of 13-(2-(4-methoxybenzilydene)-N-benzoyl-4-phenyl-oxazolidyl-)-baccatin III having the following physical and spectroscopical characteristics:
[0039]1H-NMR: 8.07 (d, Bz) 7.60-7.19 (m, aromatic), 7.48-6.90 (AA′, BB′, p-OMePh), 6.33 (s, H-10 ), 5.67 (d, J=5 Hz, H-2), 5.56 (br s, H-3′), 4.93 (d, J=8 Hz, H-5), 4.90 (brs, H-2′), 4.45 (m, H-7), 4.28 (d, J=8 Hz, H-20a), 4.16 (d, J=8 Hz, H-20b), 3.82 (s, OMe), 2.27 (s, Ac), 2.08 (s, OAc), 1.66 (s, H-19), 1.29-1.16 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com