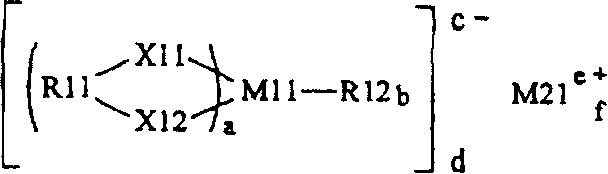Battery
A battery and electrolyte technology, applied in secondary batteries, battery electrodes, lithium batteries, etc., can solve problems such as reduced discharge capacity and reduced cycle performance, and achieve the effects of improving battery performance, preventing reactions, and improving deposition-dissolution efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1 to 1-25
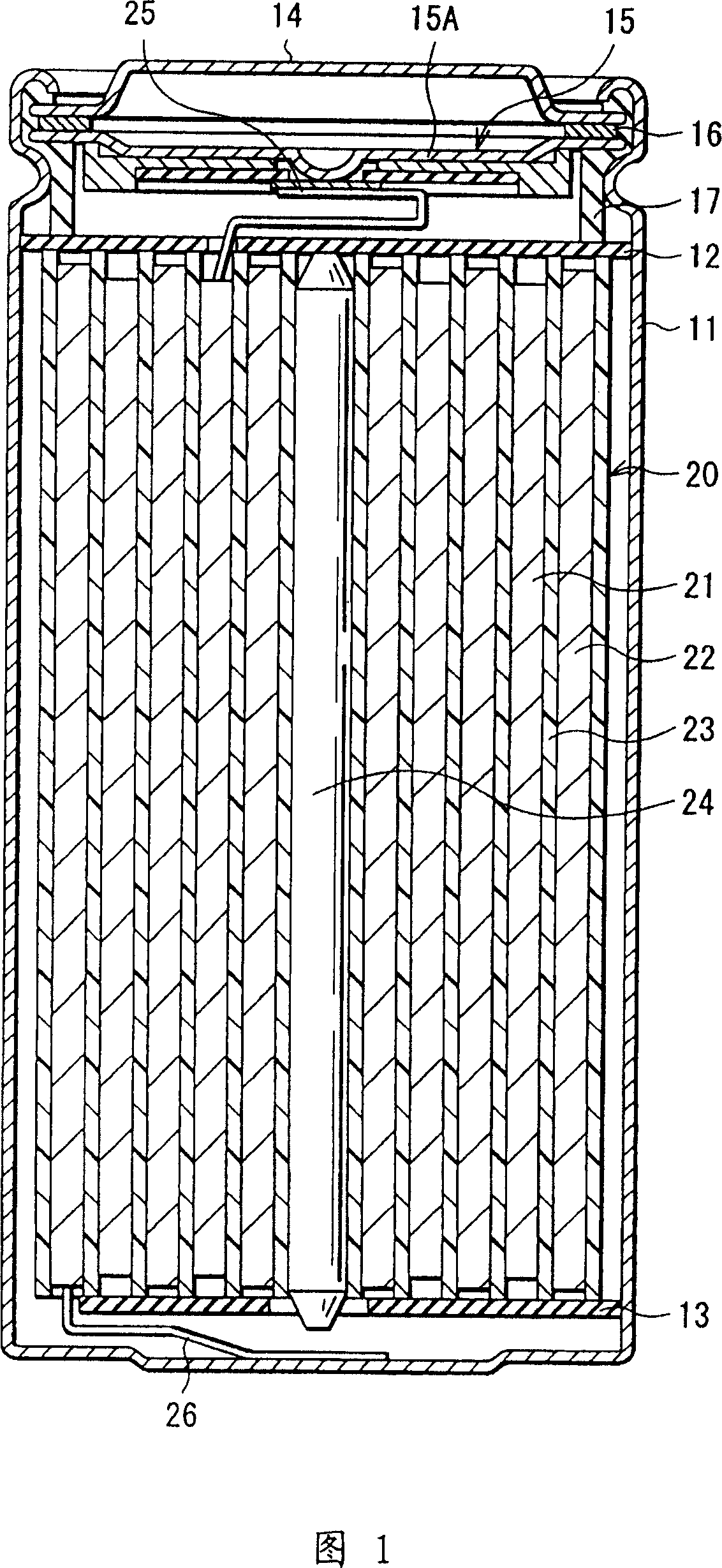
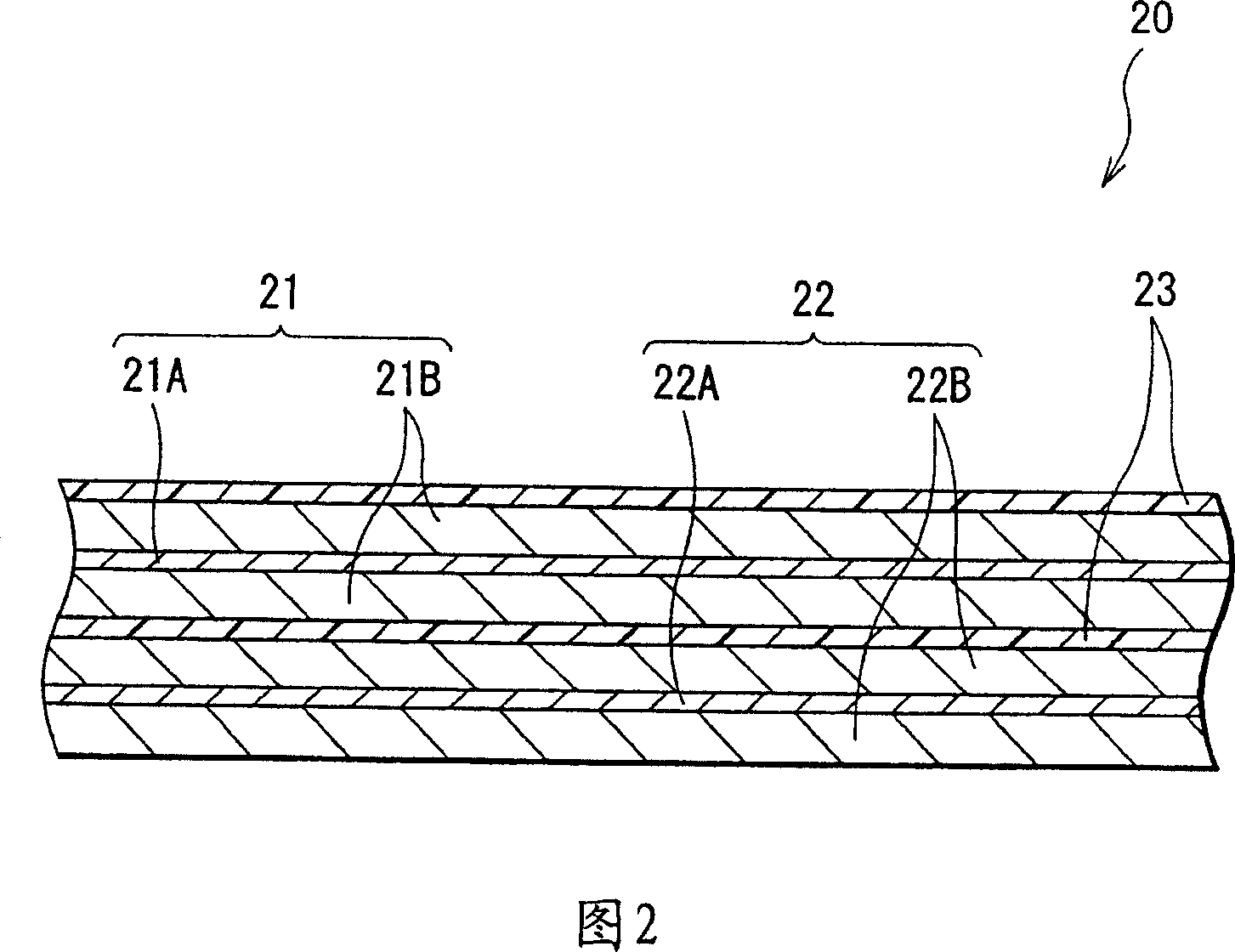
[0099] Forming a battery in which the area density ratio of the cathode 21 and the anode 22 has been adjusted, the capacity of the anode 22 includes a capacity component obtained by insertion and extraction of lithium and a capacity component obtained by deposition and dissolution of lithium, and is represented by the sum of the two .
[0100] First, by ratio (molar ratio) Li 2 CO 3 : CoCO 3 =0.5:1 mixed lithium carbonate (Li 2 CO 3 ) and cobalt carbonate (CoCO 3 ), the mixture was sintered at 900°C in air for 5 hours to obtain lithium cobalt composite oxide (LiCoO 2 ) as the cathode material. Next, 91 parts by weight of lithium cobalt composite oxide, 6 parts by weight of graphite as a conductor, and 3 parts by weight of polyvinylidene fluoride as a binder were mixed to prepare a cathode mixture. Then, the cathode mixture was dispersed in N-methyl-2-pyrrolidone as a solvent to generate cathode mixture slurry. After the cathode mixture slurry was unevenly coated on bot...
Embodiment 2-1 to 2-4
[0119] The formation of the secondary batteries of Examples 2-1 to 2-4 was basically the same as that of Examples 1-1 and 1-3, except that vinylene carbonate (VC) or vinylene carbonate was the unsaturated compound carbonate. Ethyl ester (VEC) was added to a mixture of 50% by volume ethylene carbonate and 50% by volume diethyl carbonate. The mass content of vinylene carbonate or vinyl ethylene carbonate in the solvent is 1%.
[0120] As in the case of Example 1-1, in the secondary batteries of Examples 2-1 to 2-4, cycle performance and heavy load performance were determined, and whether or not lithium metal was deposited under fully charged conditions and fully discharged conditions was examined. The results thereof are shown in Table 3 together with the results of Examples 1-1 and 1-3 and Comparative Examples 1-1 to 1-3.
[0121] shown in chemical formula 1
light metal salt
other than chemical formula 1
light metal salt
in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com