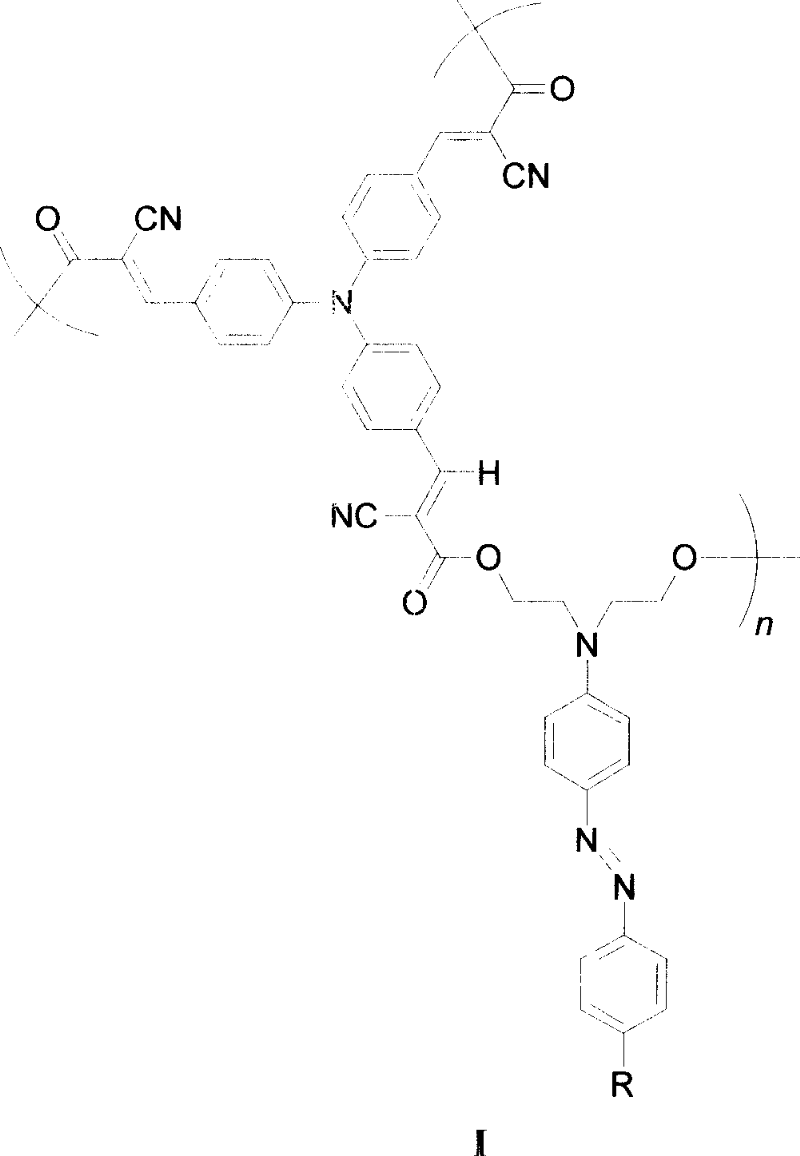
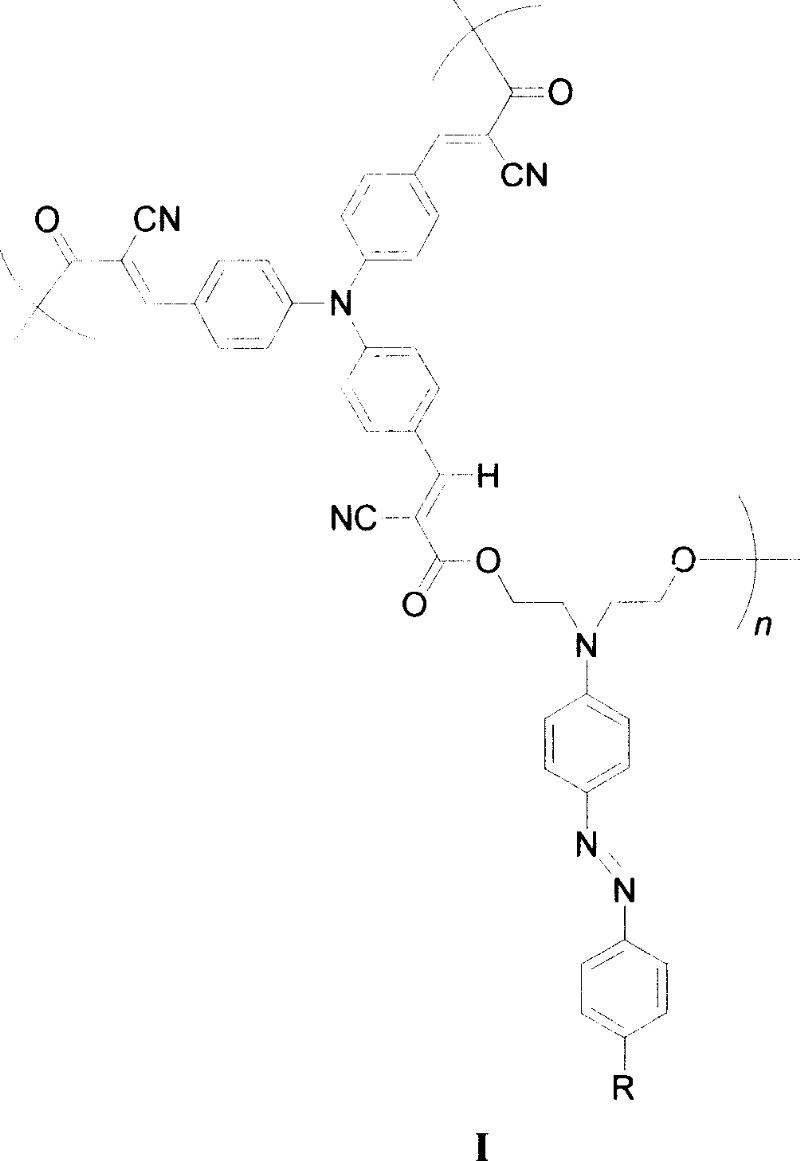
Hyperbranched second order differential non-linear optical high molecule, its preparation method and use
A second-order nonlinear, polymer technology, used in nonlinear optics, optics, instruments, etc., can solve the problems of difficult to achieve high nonlinear optical effects, difficult to achieve regular structure, complicated preparation and other problems, and achieve rich research. The content, strong innovation, and simple effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] When R is a nitro group, the synthetic route is as follows:
[0018]
[0019]
[0020] The synthesis method is:
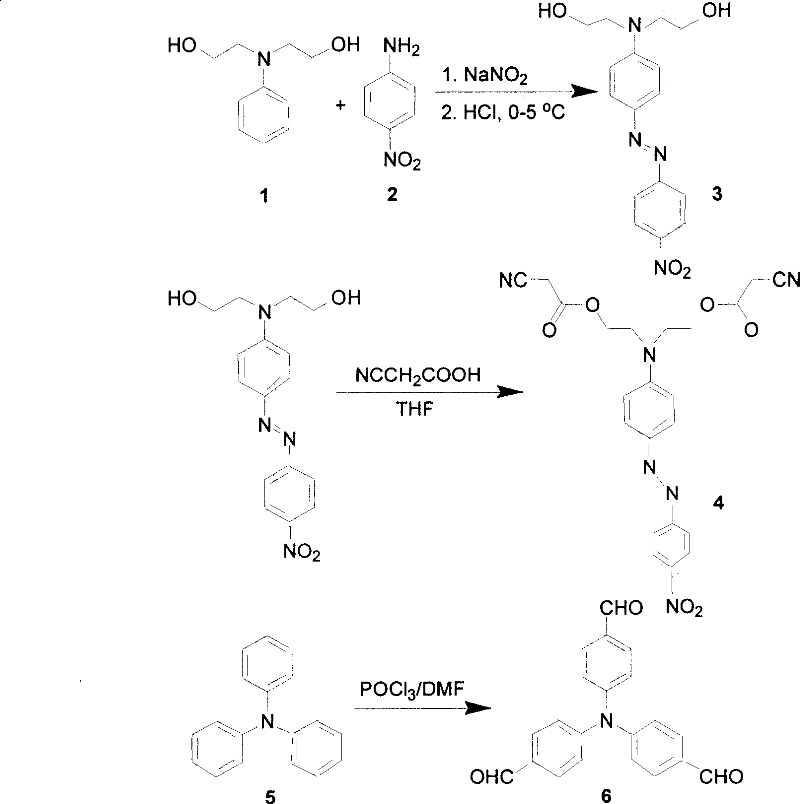
[0021] (1) Synthesis of compound 3
[0022] Add p-nitroaniline 2 to 35% aqueous solution of concentrated hydrochloric acid (the quality of water is 3 times that of concentrated hydrochloric acid), wherein the quality of concentrated hydrochloric acid is 2.5 times that of p-nitroaniline 2, and vigorously stir. Under an ice bath, slowly drop an aqueous solution of sodium nitrite into the reaction liquid, wherein the mass percent concentration of sodium nitrite is 10%-20%, keep stirring at 0° C. for 15 minutes, and then filter to remove insoluble matter. Dissolve compound 1 in appropriate ethanol and slowly add it dropwise to the vigorously stirred filtrate. After the dropwise addition, keep stirring at 0°C for 1 hour, add sodium bicarbonate to adjust the pH value to 7, and continue stirring at room temperature for half an hour. Filter and wash the filt...
Embodiment 2
[0034] When R is a sulfone group, the synthetic route is as follows:
[0035]
[0036] The synthesis method is:
[0037] (1) Synthesis of Compound 8
[0038] Add p-ethylsulfoneaniline 7 to 35% aqueous solution of concentrated hydrochloric acid (the quality of water is 3 times that of concentrated hydrochloric acid), wherein the quality of concentrated hydrochloric acid is 2.5 times that of p-ethylsulfoneaniline 7, and vigorously stir . Under an ice bath, slowly add an aqueous solution of sodium nitrite dropwise to the reaction liquid, wherein the mass percent concentration of sodium nitrite is 10%-20%, keep stirring at 0° C. for 15 minutes, and then filter to remove insoluble matter. Dissolve compound 1 in appropriate ethanol and slowly add it dropwise to the vigorously stirred filtrate. After the dropwise addition, keep stirring at 0°C for 1 hour, add sodium bicarbonate to adjust the pH value to 7, and continue stirring at room temperature for half an hour. Filter and w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com