Chinese medicinal composition for treating endometriosis menstrual ab dominal pain and its preparing method
A technology of endometrium and composition, which is applied in the field of traditional Chinese medicine composition, can solve problems such as aggravation, lowering the quality of life and health status, and achieve the effect of treating abdominal pain during menstruation, having no toxic side effects, and promoting the recovery of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
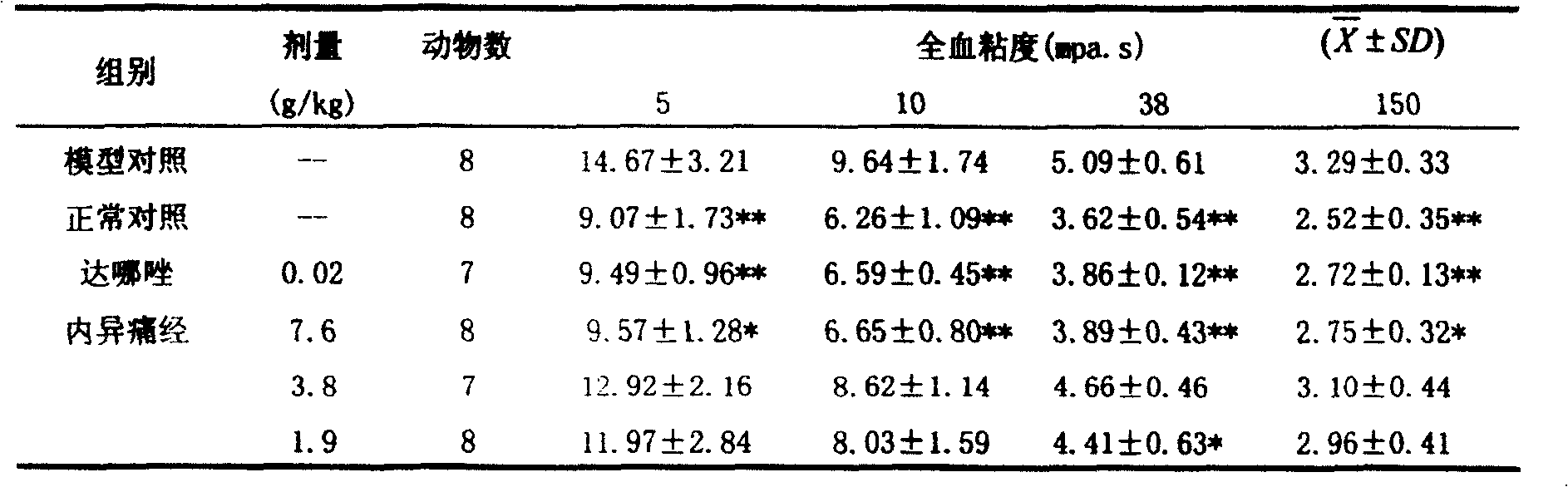
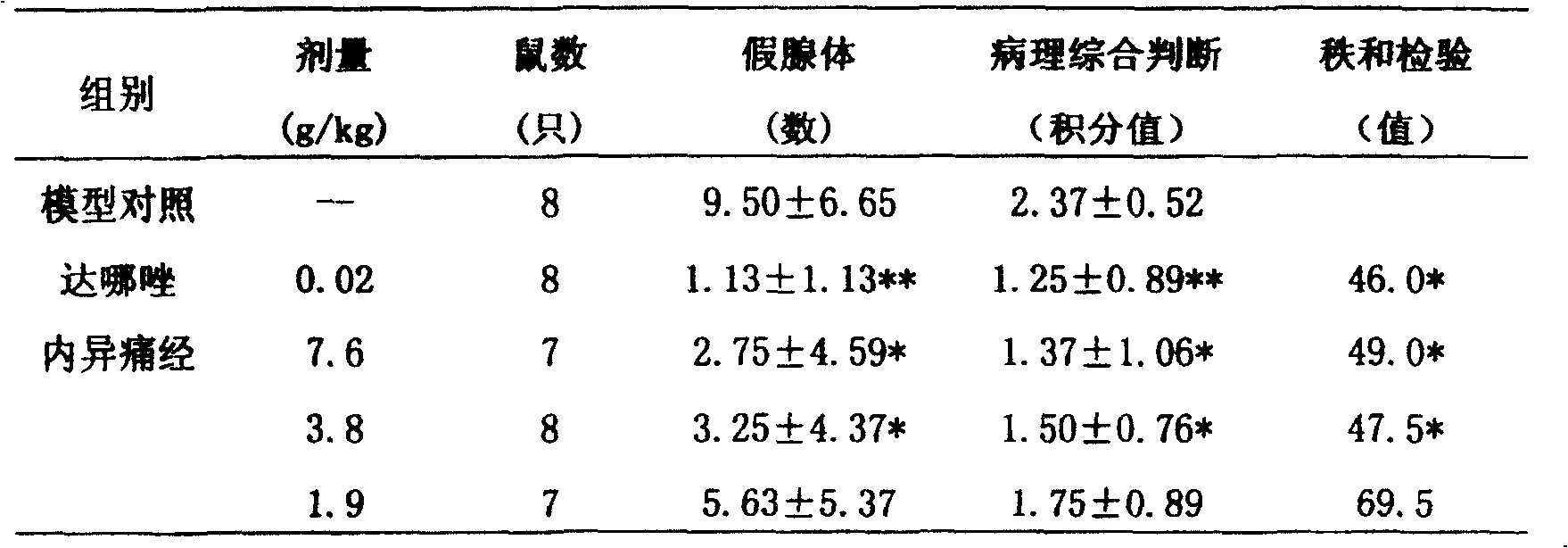
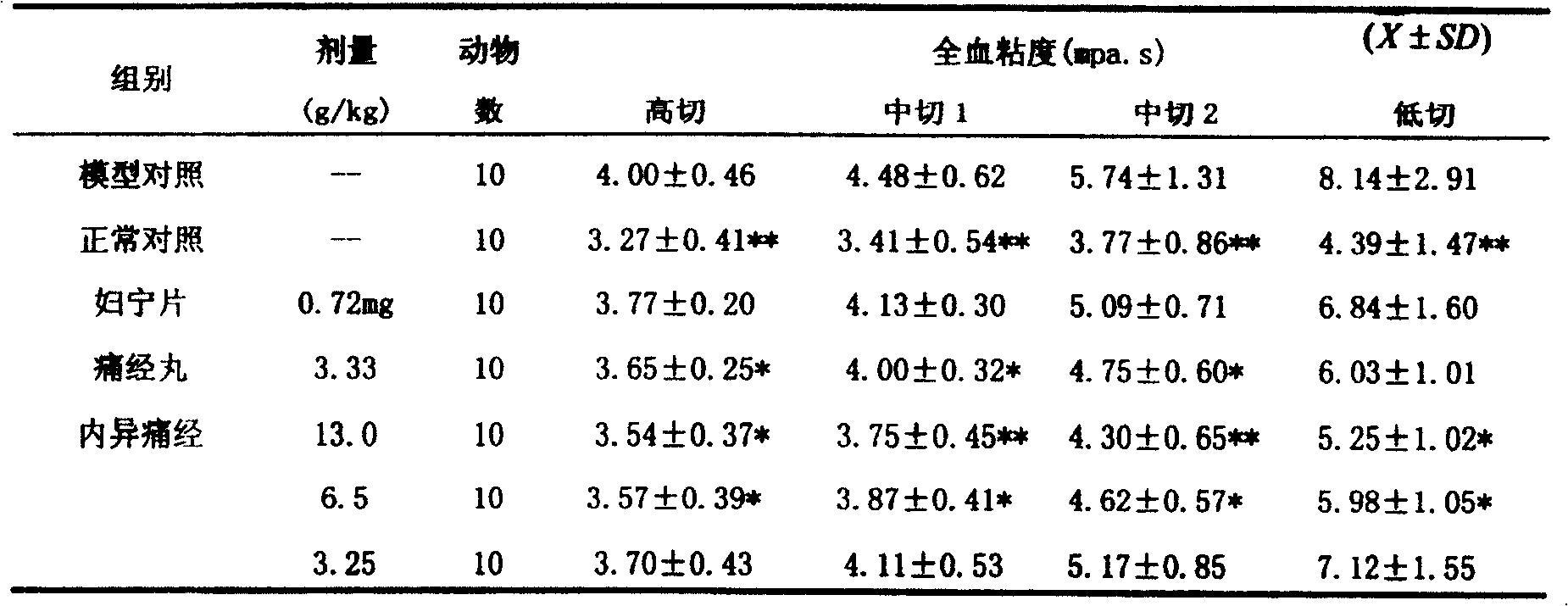
[0040] Experimental example 1 Pharmacodynamic experiment: The composition preparation of the present invention has the functions of removing blood stasis and relieving pain, dehumidifying and dispelling stagnation, promoting body recovery, and treating menstrual abdominal pain caused by endometriosis through experiments.
[0041] 1. Main pharmacodynamic research
[0042] Test drug: Neiyitongjing Granules, provided by Shanxi Bao Pharmaceutical Group Co., Ltd., batch number 020627, containing 8.5g crude drug / g powder, prepared with distilled water to the required concentration before use. Dosage design: The clinical dosage is 72g crude drug / d, which is 1.2g crude drug / kg / d based on 60kg calculation. Converted according to the equivalent dose of animal and human kilogram body weight, mice use 13.0g / kg / d (11 times), rats use 6.5g / kg / d (5.4 times), rabbits use 3.8g / kg / d (3.2 times) times), which is equivalent to the clinical equivalent dose being the middle dose, the high dose bei...
experiment example 2
[0168] Experimental Example 2 Toxicity Study
[0169] 1. Acute toxicity test: Neiyitongjing Granules were administered to mice three times a day at the maximum concentration and volume that the animals could tolerate, a total of 20 females, and the maximum dosage was 557.8g crude drug / kg , equivalent to 464.8 times the clinical dosage. The reaction of the animals was observed immediately after the administration and continued for 7 days. No death occurred, the body weight of all animals increased, and no obvious toxic reaction was observed by naked eyes. Provide reference for clinical safety drug use. 2. Long-term toxicity test: Rats were given 60.0, 30.0, and 15.0 g of crude drug / kg / d of Neiyitongjing Granules respectively by intragastric administration (equivalent to 50.0, 25.0, and 12.5 times the clinical dosage of humans) for 26 consecutive weeks. Observation for 30 days. There was no significant effect on the general conditions of the animals, such as weight gain and ...
experiment example 3
[0170] Experimental Example 3 Clinical Trial
[0171] Source of cases: 44 cases were observed in this group, all of which came from the Gynecology Clinic of Beijing Hospital of Traditional Chinese Medicine. Among them, 33 cases were in the observation group, and 11 cases were in the Chinese medicine control group (Tongjingbao Granules).
[0172] Comparison of the ages of the two groups
[0173] The age of the treatment group was 20-46 years old, with an average age of 33.42±4.17 years old.
[0174] The age of the control group was 20-44 years old, with an average age of 33.18±7.04 years old.
[0175] Comparison of disease course between the two groups
[0176] The course of disease in the treatment group ranged from 1 to 20 years, with an average of 7.82±3.43 years.
[0177] The course of disease in the control group ranged from 1 to 16 years, with an average of 5.83±2.82 years.
[0178] Comparison of disease stages between the two groups
[0179] Treatment group: 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com