Use of green chiretta diterpene lactone in inhibiting vascularization
A technology of andrographis diterpene lactone and diterpene lactone is applied in the new application field of andrographis diterpene lactone, which can solve the problem that the anti-cancer mechanism of active ingredients is unclear, the anti-cancer effect is limited, the dosage is unreasonable, and the like. problems, to achieve the effect of not easy drug resistance, high curative effect, and stable expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
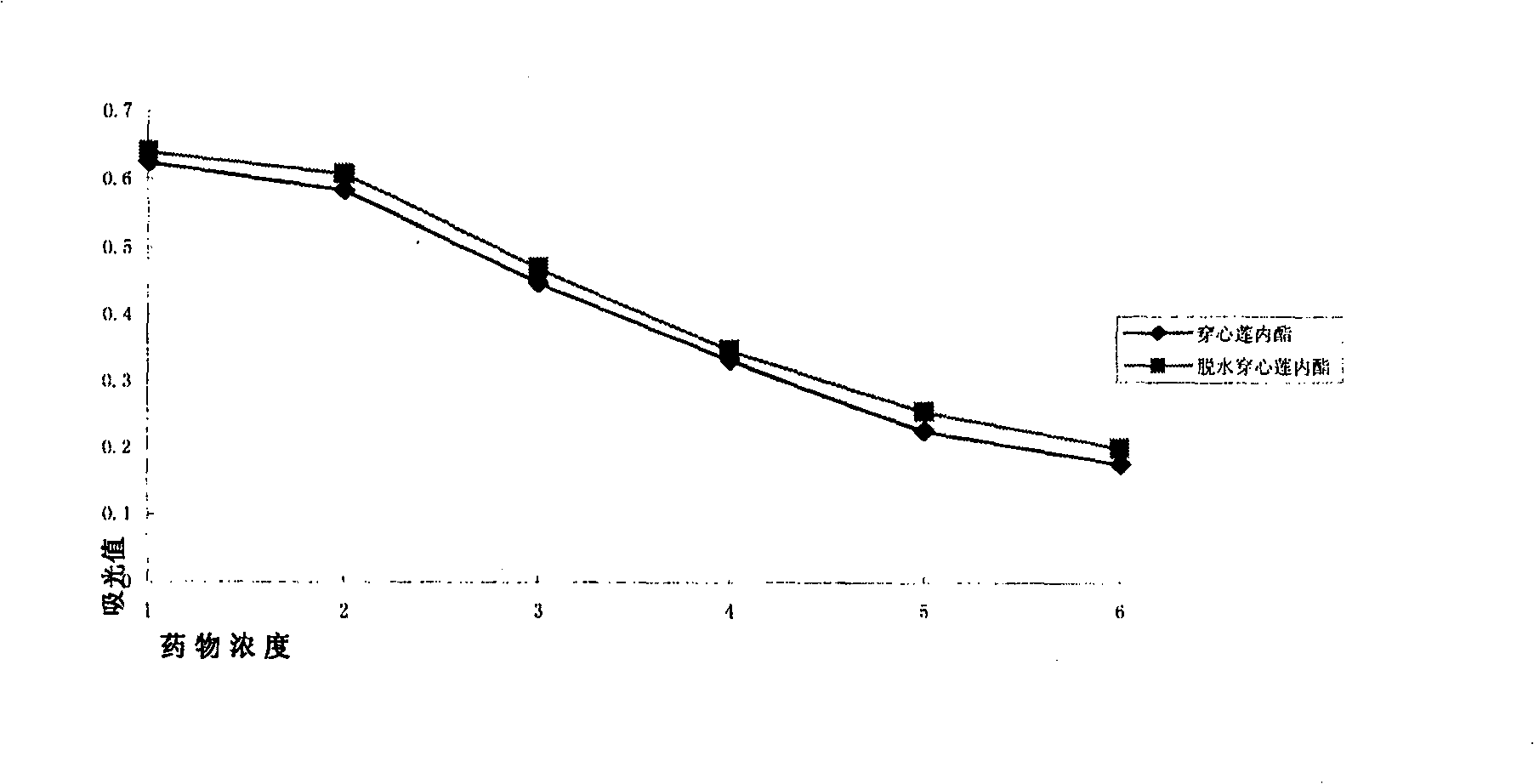
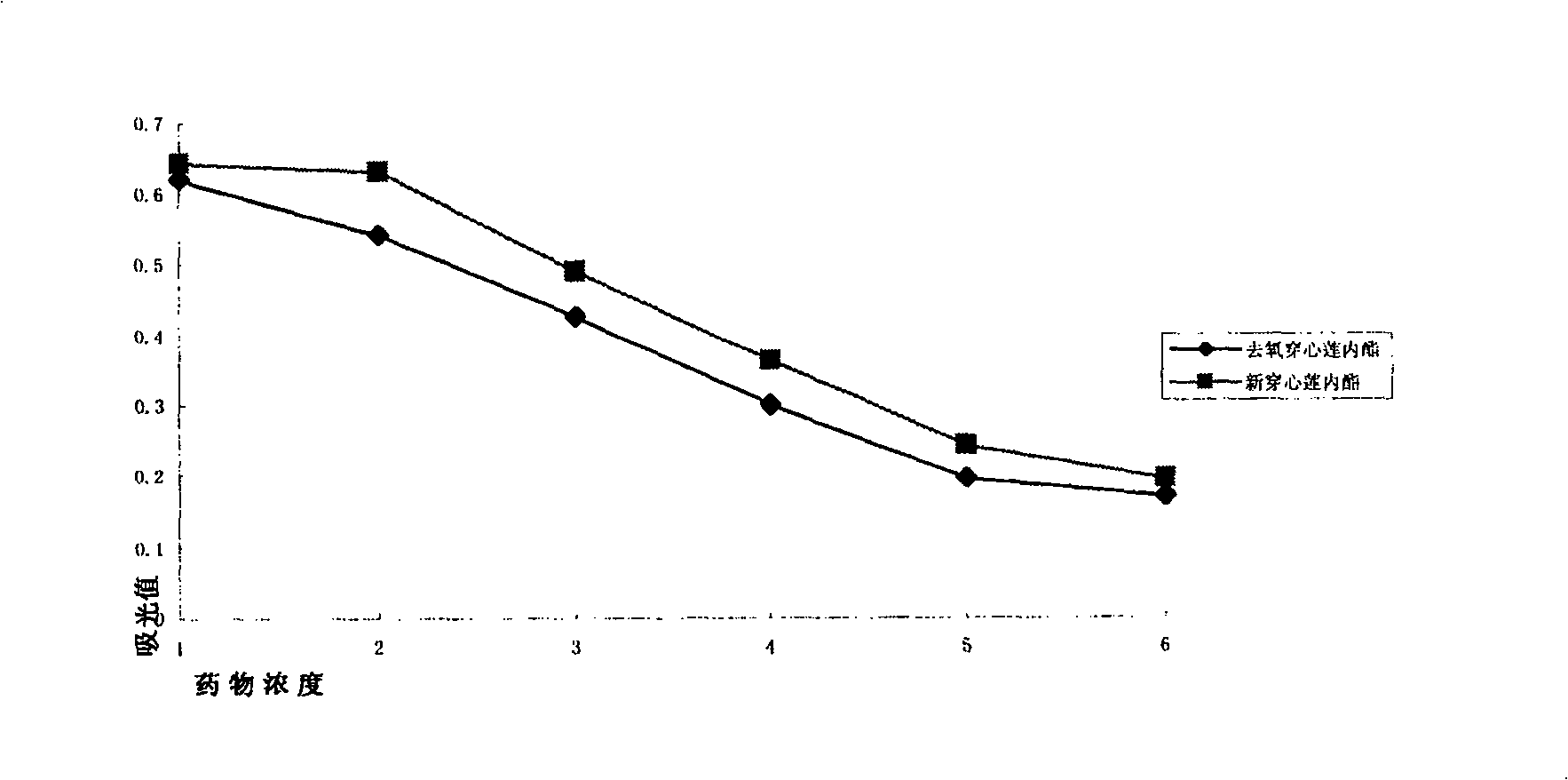
[0031] Example 1: The inhibitory effect of andrographis paniculata diterpene lactone on the proliferation of human vascular endothelial cells.
[0032] Human umbilical vein endothelial cells (HUVEC, purchased from Cascade Biologics, Cot: C-003-5C) were cultured in Medium 200 (purchased from Cascade Biologics) containing 10% fetal bovine serum (FBS) (37°C, 5%CO 2 , 95% humidity) culture, the fourth-generation cells were inoculated in a 96-well culture plate at a density of 4000 / 250ul, and a control group, a drug group and a blank control group with various concentration gradients were set, and 3 replicate holes were made in each group. When the cell growth density reaches 80%, add 5ul of four kinds of andrographolide diterpene lactone compounds (i.e.: andrographolide, dehydroandrographolide, deoxyandrographolide, neoandrographolide) of each concentration gradient respectively, all dissolved with DMSO ), so that the final concentration was 1000uM, 100uM, 10uM, 5uM and 1uM respe...
Embodiment 2
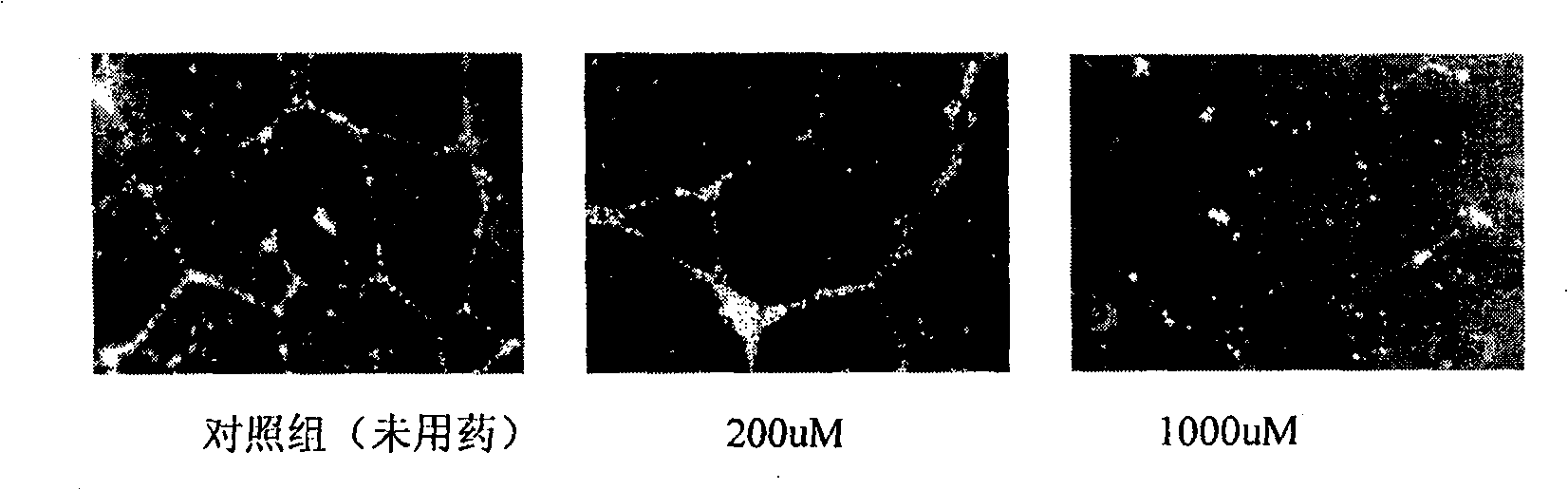
[0033] Example 2: Inhibitory effect of andrographis paniculata diterpene lactone on tube formation ability of human vascular endothelial cells.
[0034] Add BD Matrigel to each well of a 24-well culture plate TM Matrix original glue 200ul, make it polymerized into gel, the fourth generation human umbilical vein endothelial cell (HUVEC) suspension was inoculated into the 24-well plate coated with Matrigel glue at a density of 30000cell / 500ul, set the control group, each concentration gradient The drug group was added with 10ul concentration gradient of andrographolide (200uM, 1000uM, dissolved in DMSO), and the control group was added with 10ul of sterile DMSO, and 3 replicate wells were made in each group. 37°C, 5% CO 2 , 95% humidity for 24 hours, OLYMPUS CK40-RFL optical microscope to observe the formation of vascular endothelial cell tubes, take 4 low-power fields from each well and count them, and take pictures with an OLYMPUS CK40 digital camera. The result is as ima...
Embodiment 3
[0035] Example 3: Inhibitory effect of andrographolide diterpene lactone on migration ability of human vascular endothelial cells.
[0036] 1. Preparation of chemokines
[0037] The NIH3T3 cells that grew well the next day after passage were used. Gently rinse with serum-free DMEM twice. Serum-free DMEM, 5% CO 2 , 37°C for 24-48 hours. Collect the cell supernatant. Centrifuge (12000g, 4°C, 10min). Filter the supernatant (0.22um filter membrane) and store in aliquots (-20°C).
[0038] 2. Matrigel invasion test
[0039] Take 25ul of diluted Matrigel (the original glue was diluted with DMEM at a ratio of 1:2) and add it to the chamber on the Transwell plate to cover the entire surface of the polyester film, and incubate at 37°C for 30min to make Matrigel polymerize into a gel. The fourth-generation human umbilical vein endothelial cell (HUVEC) suspension was inoculated into the upper chamber at a density of 30000 / 250ul, washed 3 times with PBS, digested and harvested from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com