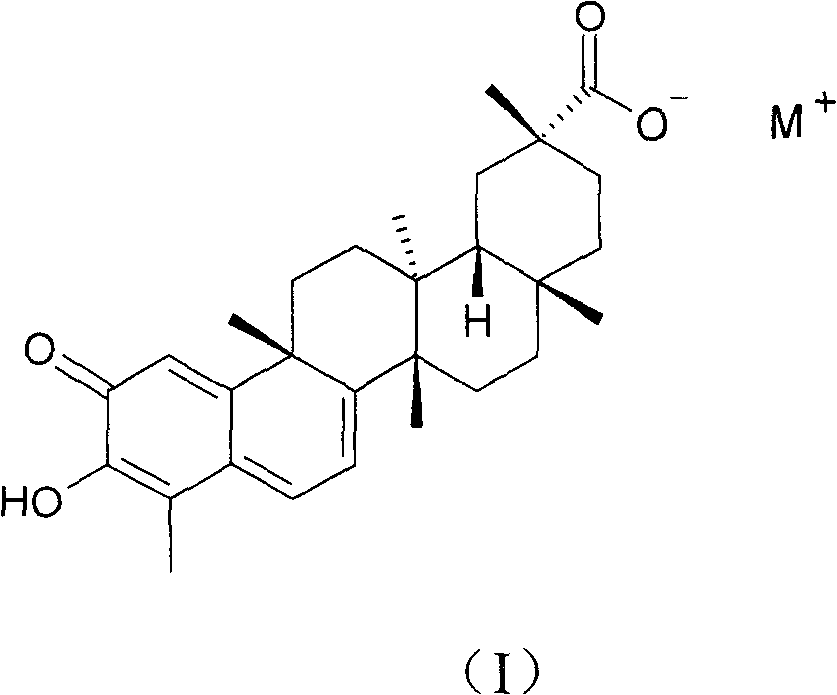
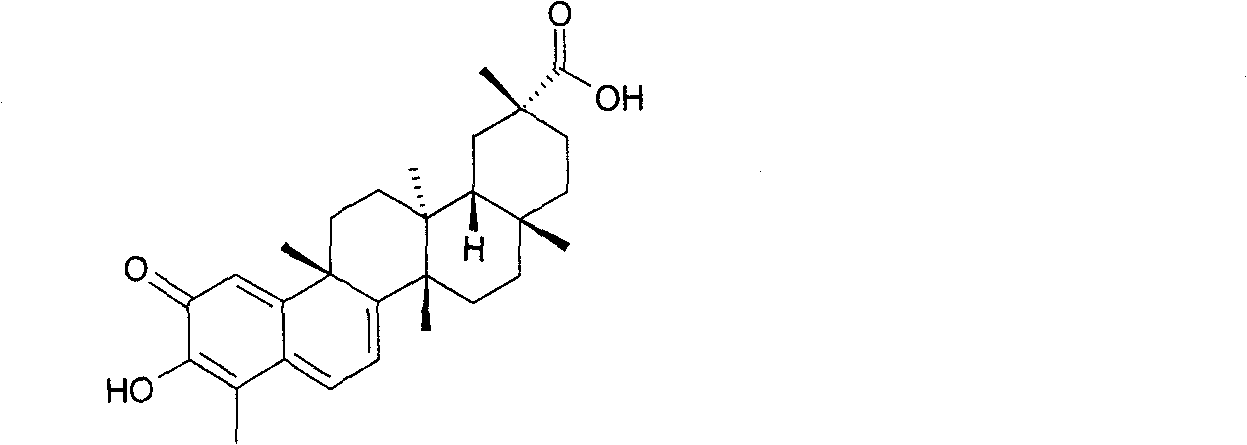
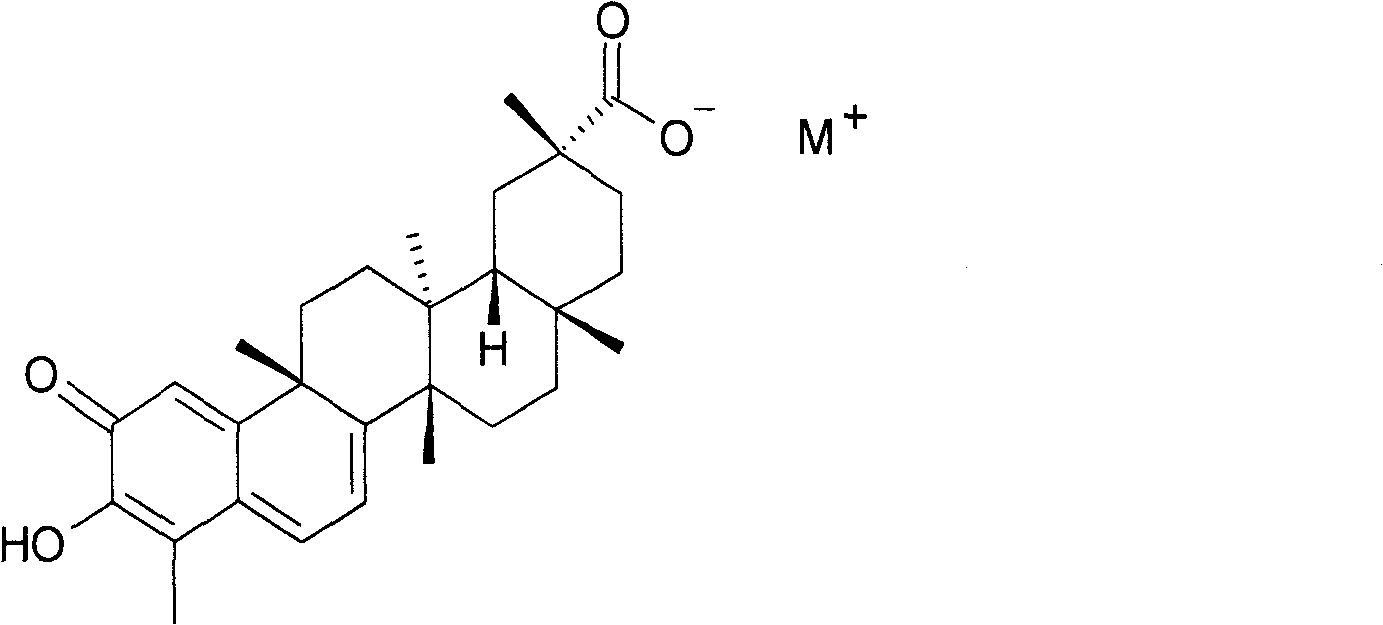
Alkali metal salt compound of celastrol and preparation method thereof
A technology of celandine and alkali metal salts, which is applied in the fields of steroids, chemical recovery, organic chemistry, etc., can solve the problems of instability, weak carboxyl acidity, and inability to use the human body, etc., and achieves the advantages of convenient operation and reduced packaging conditions Requirements, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of sherendin sodium salt compound.
[0025] After dissolving 101.5 mg of cerulein in 2-3 ml of ethanol, add 1 ml of aqueous sodium bicarbonate solution (containing 19.9 mg of sodium bicarbonate), mix well, add a small amount of water dropwise to clarify the solution, and stir at room temperature for 4 hours. Concentrate under reduced pressure at 40°C to dryness, add 4ml of absolute ethanol to the residue to dissolve, filter, add ethyl acetate dropwise to the filtrate until cloudy, refrigerate at 5°C for 12 hours, a dark red precipitate precipitates, filter it out, and dry under reduced pressure at 40°C for 3 hours, the product 92mg was obtained. Yield: 75.8%.
[0026] Analysis results:
[0027] The H-NMR spectrum data of ceherin sodium salt: 1 H NMR (DMSO, 300M), δ7.052 (2H, d, J=7.2Hz, H-6), 6.367 (1H, s, H-1), 6.315 (1H, d, J=7.2Hz, H- 7), 2.083 (3H, s, CH 3 -23), 1.362, 1.198, 1.016, 0.922, (each 3H, s, CH 3 -25,CH 3 -26,CH 3 -30,CH 3 ...
Embodiment 2
[0035] Example 2: Preparation of sherendin potassium salt compound.
[0036] After dissolving 100.9 mg of cerulein in 2-3 ml of methanol, add 1 ml of aqueous potassium bicarbonate solution (containing 23.5 mg of potassium bicarbonate), mix well, add a small amount of water dropwise to clarify the solution, and stir at room temperature for 4 hours. Concentrate to dryness under reduced pressure at 40°C, add 4ml of absolute ethanol to the residue to dissolve, filter, and concentrate the filtrate to dryness under reduced pressure at 35°C to obtain a dark red powder, dry under reduced pressure at 35°C for 3 hours to obtain 97mg of the product. Yield: 78.8%.
[0037] Analysis results:
[0038] The H-NMR spectrum data of ceherin potassium salt: 1H NMR (DMSO, 300M), δ7.053 (2H, d, J=7.2Hz, H-6), 6.373 (1H, s, H-1), 6.319 (1H, d, J=7.2Hz, H- 7), 2.085 (3H, s, CH 3 -23), 1.362, 1.194, 1.008, 0.872, (each 3H, s, CH 3 -25, CH3-26, CH 3 -30,CH 3 -28), 0.720 (3H, s, CH 3 -27).
[0...
Embodiment 3
[0040] Example 3: Preparation of ceherin sodium salt compound.
[0041] After dissolving 10.23g of shertenin in 200ml of methanol, add 100ml of aqueous sodium bicarbonate solution (containing 2.00g of sodium bicarbonate), mix well, add a small amount of water dropwise to clarify the solution, and stir at room temperature for 4 hours. Concentrate under reduced pressure at 40°C to dryness, add 200ml of isopropanol to the residue to dissolve, filter, add ethyl acetate dropwise to the filtrate until cloudy, refrigerate at 0°C for 12 hours, a deep red precipitate is obtained, filter it out, and dry under reduced pressure at 40°C After 5 hours, 10.52 g of the product was obtained. Yield: 86%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| process yield | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com