Styrene derivative, styrene polymer, photosensitive resin composition, and method for forming pattern
A technology of styrene polymer and resin composition, applied in the field of new styrene derivatives, can solve problems such as safety and environmental problems, and achieve the effect of increasing solubility difference and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
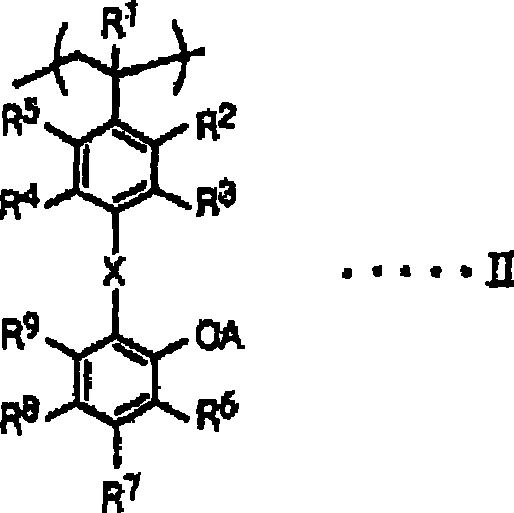
[0168] Synthesize a styrene derivative having the following structure, that is, a styrene derivative represented by general formula I, wherein, R 1 —R 9 is a hydrogen atom, X is -CONH-, and A is a hydrogen atom.
[0169]
[0170] 50 g of 4-vinylbenzoic acid and 62.1 g of pentafluorophenol were dissolved in a mixed solvent of 500 mL of ethyl acetate and 150 mL of tetrahydrofuran, and the solution was cooled with ice. 69.65 g of dicyclohexylcarbodiimide was added to the above solution, and the mixture was stirred under ice-cooling for 1 hour and then at room temperature for 1 hour. The precipitated dicyclohexylurea was filtered off, and the filtrate was concentrated under reduced pressure. 150 mL of heptane was added to the residue, and the precipitated dicyclohexylurea was filtered off. Then, the filtrate was concentrated under reduced pressure to obtain 99 g of pentafluorophenyl 4-vinylbenzoate.
[0171] Then, 10 g of pentafluorophenyl 4-vinylbenzoate and 4.17 g of orth...
Embodiment 2
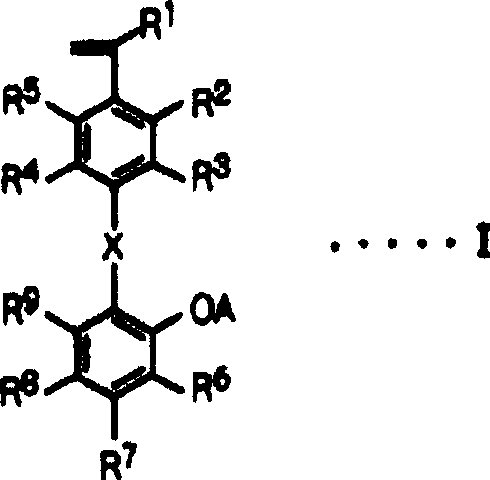
[0174] Synthesize a styrene derivative having the following structure, that is, a styrene derivative represented by general formula I, wherein, R 1 —R 9 is a hydrogen atom, X is -CH=N-, and A is a hydrogen atom.
[0175]
[0176] 5 g of 4-vinylbenzaldehyde and 4.54 g of o-aminophenol were dissolved in 100 mL of toluene, and the solution was stirred at 80-85° C. for 4 hours. After cooling the solution, the solution was concentrated to half under reduced pressure, and the precipitated ortho-aminophenol was filtered off. The residue was recrystallized from hexane / toluene (4 / 1) to obtain 5.42 g of the desired product (yield: 64%).
[0177] of the resulting compound 1 H-NMR (THF-d 8 ) results are as follows: δ 5.38 (1H, d), 5.87 (1H, d), 6.77 (1H, dd), 6.91 (1H, t), 7.02 (1H, d), 7.18—7.32 (3H, m), 7.52 (2H,d), 7.88(1H,d), 8.68(1H,s).
Embodiment 3
[0179] Synthesize a styrene derivative having the following structure, that is, a styrene derivative represented by general formula I, wherein, R 1 —R 9 is a hydrogen atom, X is -CONH-, and A is ethoxymethyl.
[0180]
[0181] 10 g of the styrene derivative obtained in the examples and 8.1 g of N-ethyldiisopropylamine were dissolved in 90 mL of N-methyl-2-pyrrolidone. 4.346 g of chloromethyl ethyl ether was added to the solution, and the mixture was reacted at room temperature for 20 hours. 200 mL of diethyl ether was added to the reaction solution, and the mixture was washed successively with 0.2N hydrochloric acid, 3% aqueous sodium bicarbonate solution and brine. The organic layer was dried over magnesium sulfate and evaporated under reduced pressure. The residue was recrystallized from hexane to obtain 8.76 g of the desired styrene derivative (yield: 70%).
[0182] of the resulting compound 1 H-NMR (THF-d 8 ) results are as follows: δ 1.19 (3H, t), 3.74 (2H, q), 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com