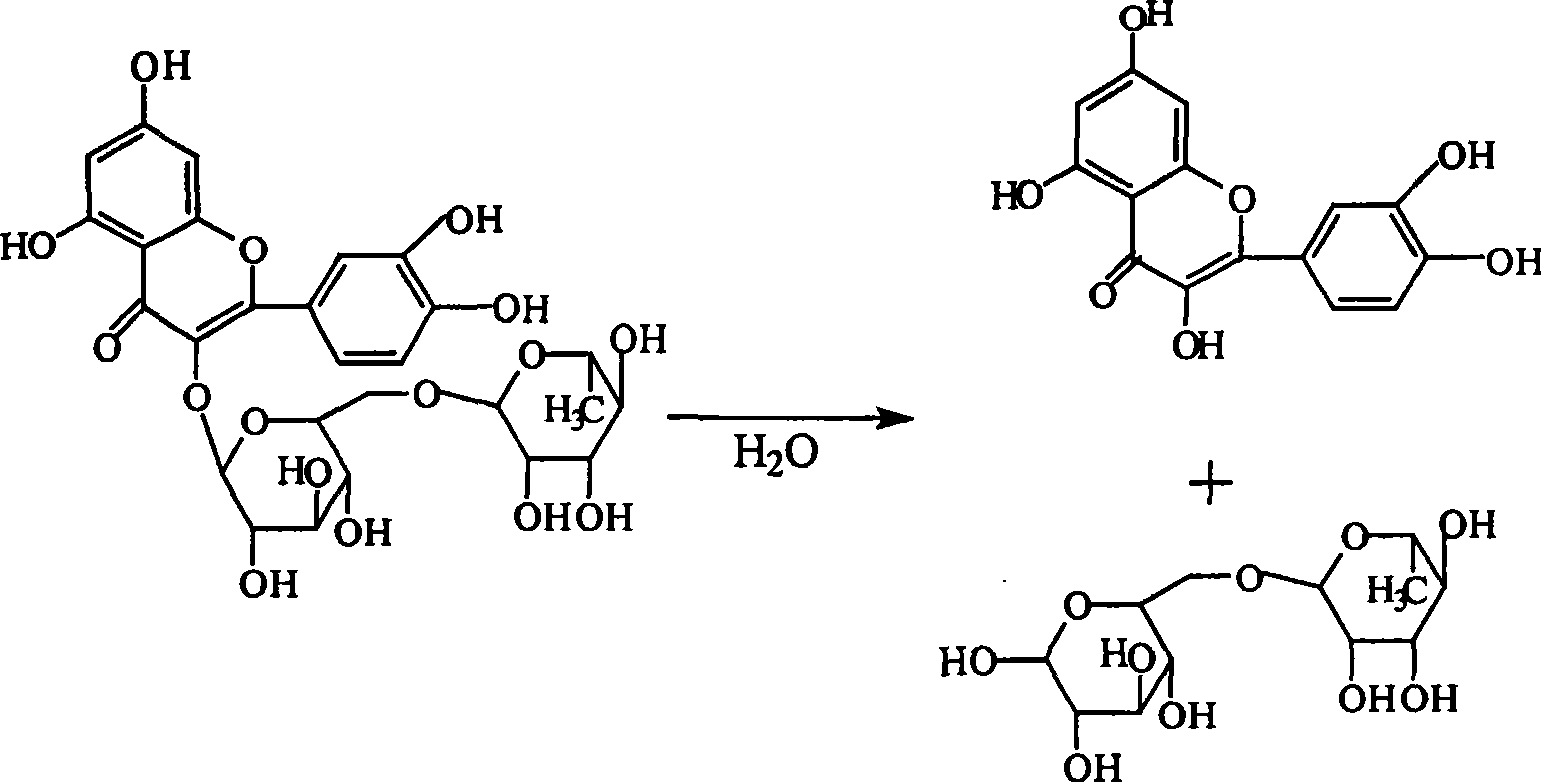
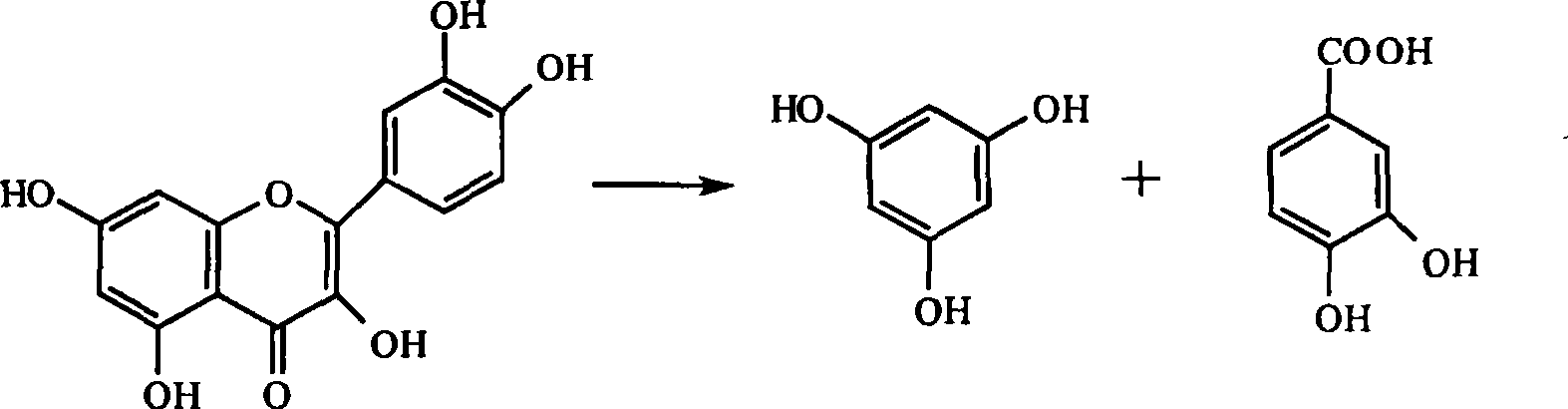
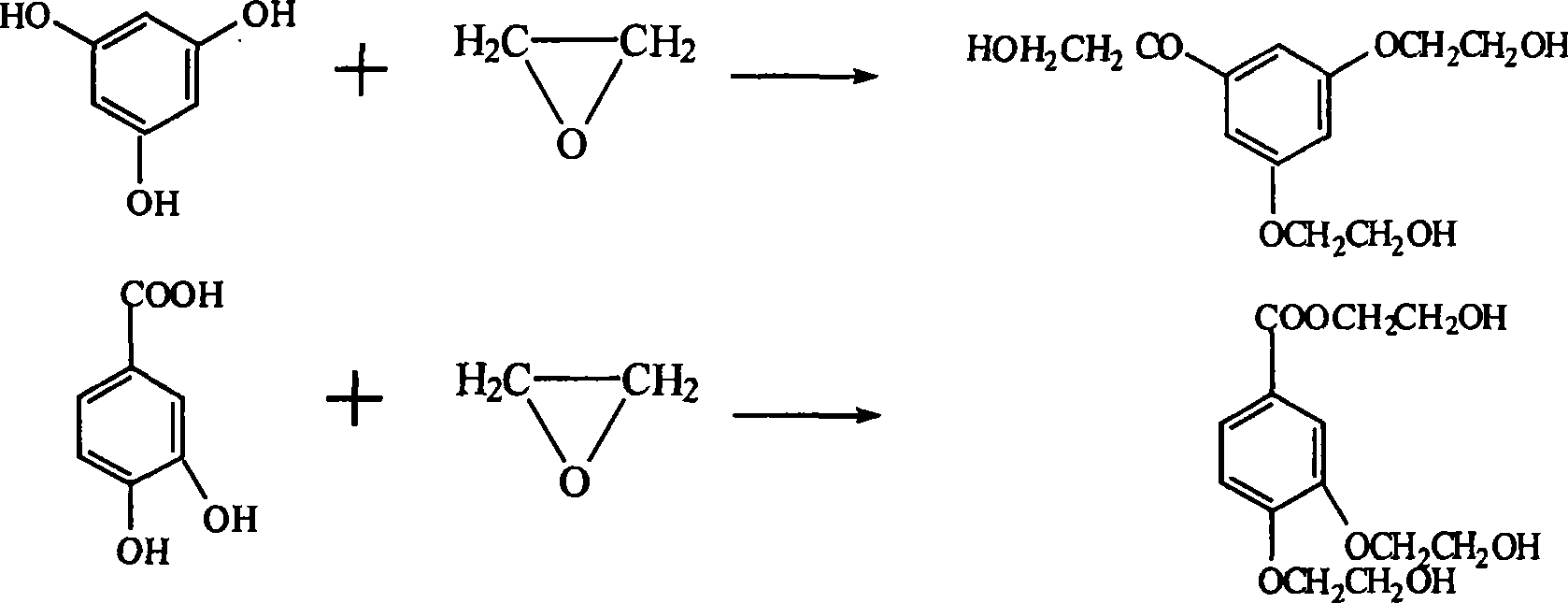
Method for preparing high-content troxerutin drug
A technology of rutin and trihydroxyethyl rutin, which is applied in the field of preparation of high-content troxerutin medicines, can solve the problems that troxerutin does not have pharmacological effects, shorten reaction time, increase reaction concentration, reduce The effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the method for preparing troxerutin
[0030] At 75°C, add 100g of rutin, 100g of water, and 0.82g of sodium hydroxide into the reaction kettle, feed nitrogen to drive off oxygen; then feed ethylene oxide, monitor the pH of the solution, when the pH of the reaction solution is 9.1 , lower the reaction temperature to 60° C., add 10 g of resin, and continue the reaction until the pH value of the reaction solution is 10.0, then stop adding ethylene oxide. Add 1:1 hydrochloric acid aqueous solution to adjust the pH value of the solution to about 4.5, hot filter, concentrate, and vacuum-dry to obtain 120.5 g of troxerutin, of which the content of 3', 4', 7-trihydroxyethyl rutin detected by HPLC is 92.8%. .
Embodiment 2
[0031] Embodiment 2: the method for preparing troxerutin
[0032] At 75°C, add 100g of rutin, 150g of water, and 0.83g of sodium hydroxide into the reaction kettle, and then feed ethylene oxide to monitor the pH of the solution. When the pH of the reaction solution is 9.2, lower the reaction temperature to At 60°C, 10 g of resin was added, and the reaction was continued until the pH value of the reaction solution was 10.1, then the addition of ethylene oxide was stopped. Add 1:1 hydrochloric acid aqueous solution to adjust the pH value of the solution to about 4.5, hot filter, concentrate, and vacuum-dry to obtain 121.2 g of troxerutin, of which the content of 3', 4', 7-trihydroxyethyl rutin detected by HPLC is 85.2%. .
Embodiment 3
[0033] Embodiment 3: the method for preparing troxerutin
[0034]At 75°C, add 100g of rutin, 200g of water, and 0.85g of sodium hydroxide into the reaction kettle, and then feed ethylene oxide to monitor the pH value of the solution. When the pH value of the reaction solution is 9.3, reduce the reaction temperature to At 60°C, 10 g of resin was added, and the reaction was continued until the pH value of the reaction solution was 10.2, then the addition of ethylene oxide was stopped. Add 1:1 hydrochloric acid aqueous solution to adjust the pH value of the solution to about 4.5, hot filter, concentrate, and vacuum-dry to obtain 120.4 g of troxerutin, of which the content of 3', 4', 7-trihydroxyethyl rutin detected by HPLC is 81.6%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com