Method for preparing Tengchasu dispersibletablet and its use
A technology for dispersible tablets and rattan tea, which is applied to medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of poor clinical effect, low oral bioavailability, low water solubility, etc. Main drug solubility, easy oral administration, and convenient taking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
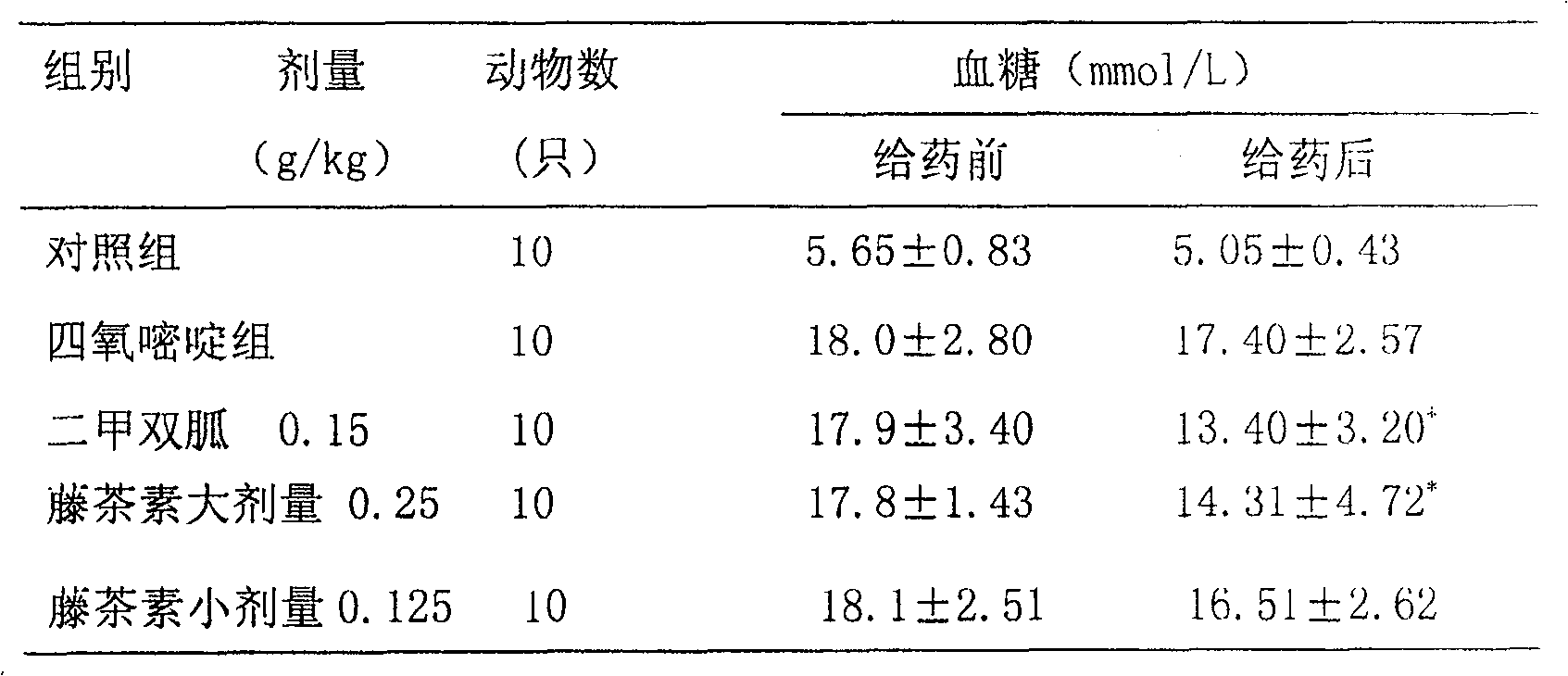
[0052] Example 1: Preparation of vine tea dispersible tablets for treating or preventing hyperlipidemia and hyperglycemia.
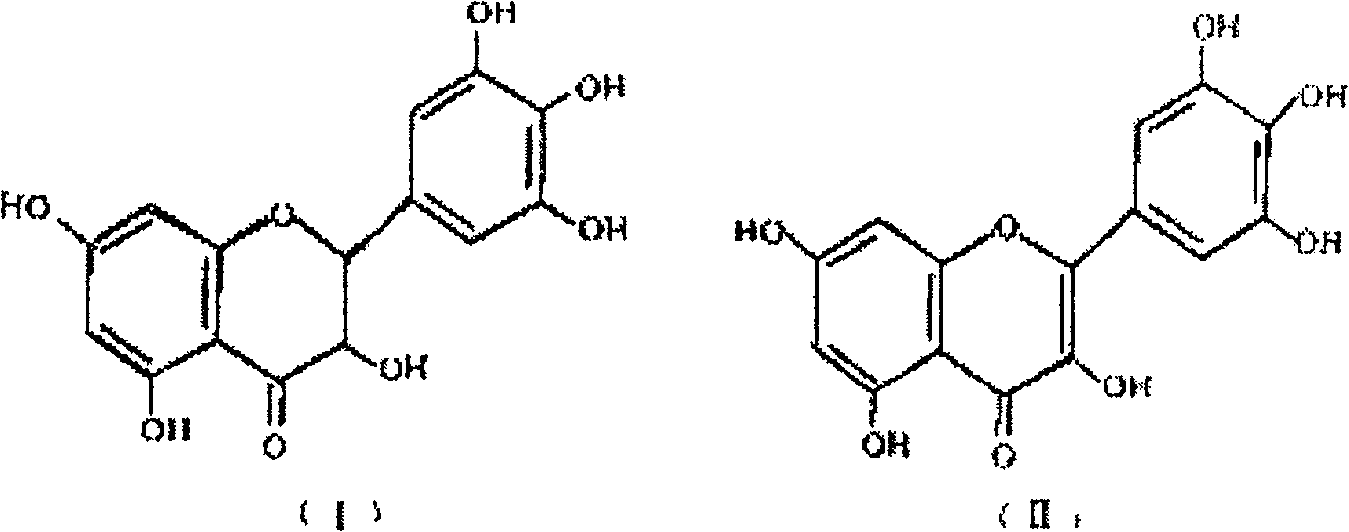
[0053] Take 3000g of processed vine tea, add 12 times the amount of 50% ethanol aqueous solution, reflux extraction for 3 times, filter, combine the extracts, boil, concentrate at pH 6-8 to a volume equivalent to 5 times the amount of medicinal materials, and place to crystallize The crystallization is carried out through ethanol-water recrystallization to obtain the total flavonoid extract of vine tea containing 70-95% of dihydromyricetin and 5-30% of myricetin.
[0054] According to the calculation of preparing 1000 tablets, weigh 230g of the above extract, 75g of microcrystalline cellulose, 48g of cross-linked polyvinylpyrrolidone, 18g of povidone K3018g, 6g of micropowder silica gel, 40g of xylitol, 11g of mannitol, and 105g of pregelatinized starch , sodium lauryl sulfate 5g, essence 2g. Combine the extract with 90-10% cross-linked polyvinylpyrroli...
Embodiment 2
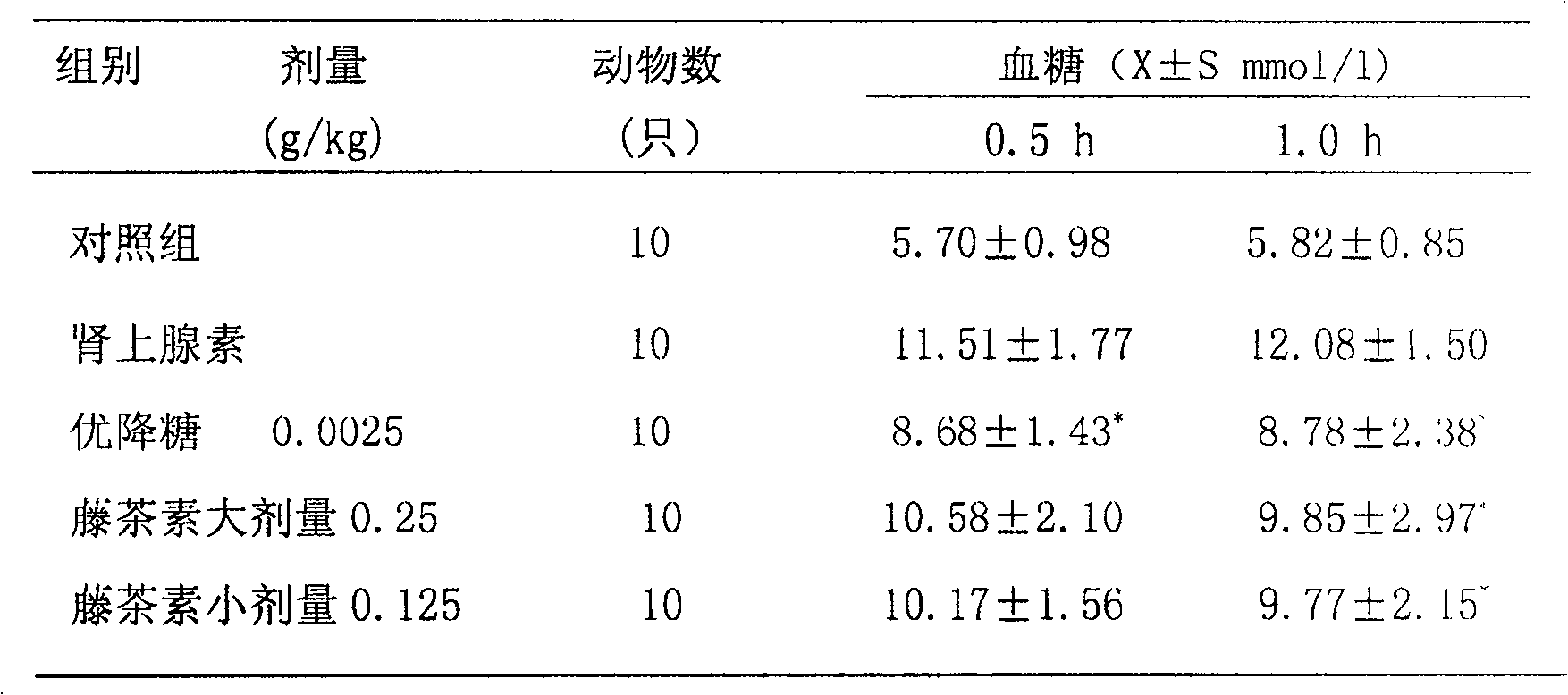
[0058] Example 2: Preparation of vine tea dispersible tablets for treating or preventing thromboembolic cardiovascular and cerebrovascular diseases.
[0059] Take 3000g of processed vine tea, add 8 times the amount of water, decoct twice, combine the extracts, boil, concentrate at pH 6-8 to a volume equivalent to 4 times the amount of medicinal materials, place for crystallization, and crystallize through ethanol- Water recrystallization can obtain the total flavonoid extract of rattan tea containing 5-70% of dihydromyricetin and 30-95% of myricetin.
[0060] According to the calculation of preparing 1000 tablets, weigh 230g of the above extract, 27g of sodium carboxymethyl starch, 28g of low-substituted hydroxypropyl cellulose, 72g of microcrystalline cellulose, 41g of sorbitol, 9g of mannitol, and 17g of povidone K30 , 110g of pregelatinized starch, 6g of micropowdered silica gel, 8g of sodium lauryl sulfate, and 2g of essence. Combine the extract with 90-10% sodium carboxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com