Preparation process of clopidogre and its salt
A clopidogrel salt and a technology for clopidogrel are applied in the field of preparing clopidogrel and its pharmaceutically acceptable salts, which can solve the problems of affecting workers' health, labor consumption, energy, incomplete racemization and the like, and save operation time. and labor costs, shortening the reaction cycle, simple and time-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
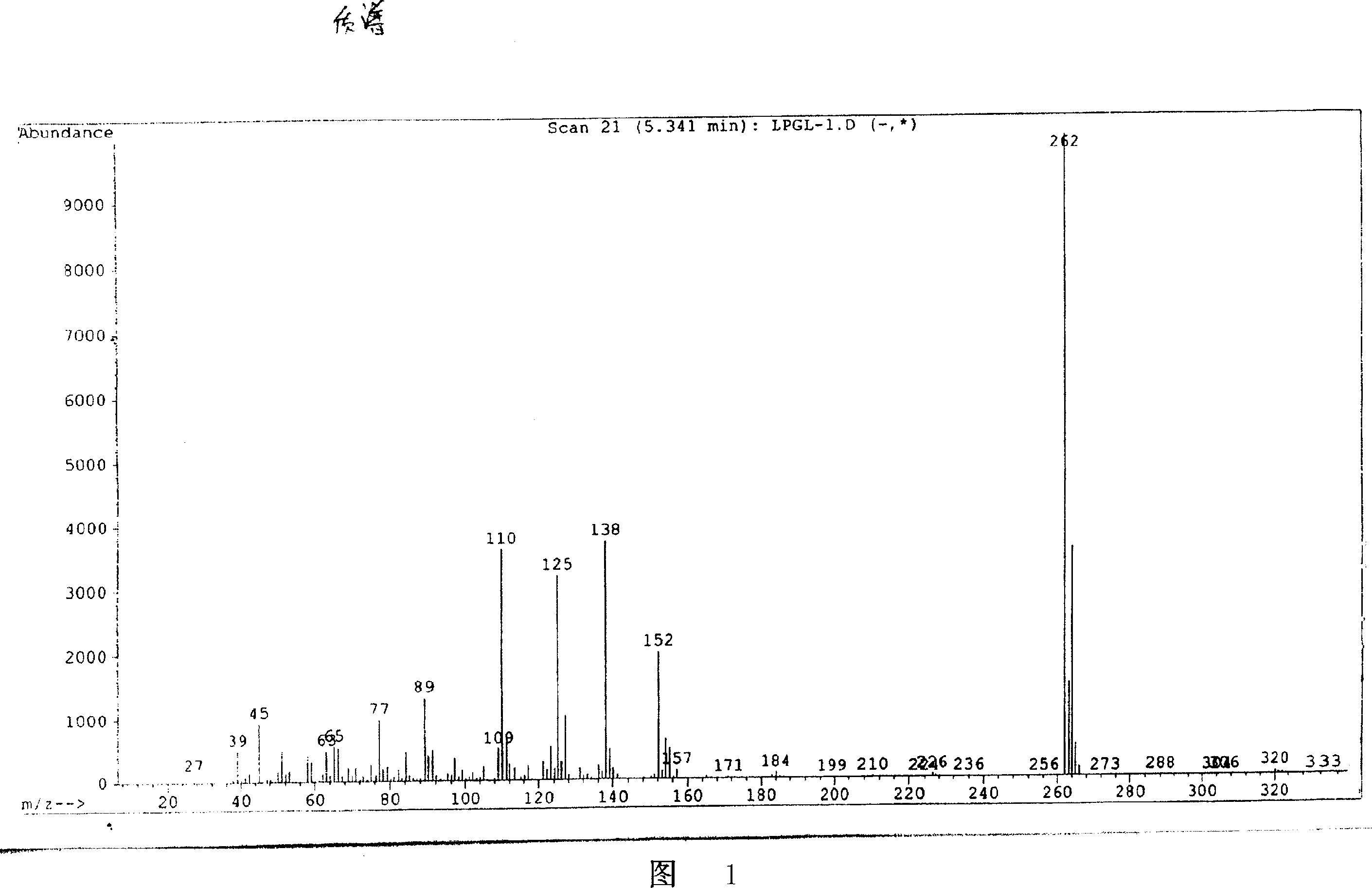
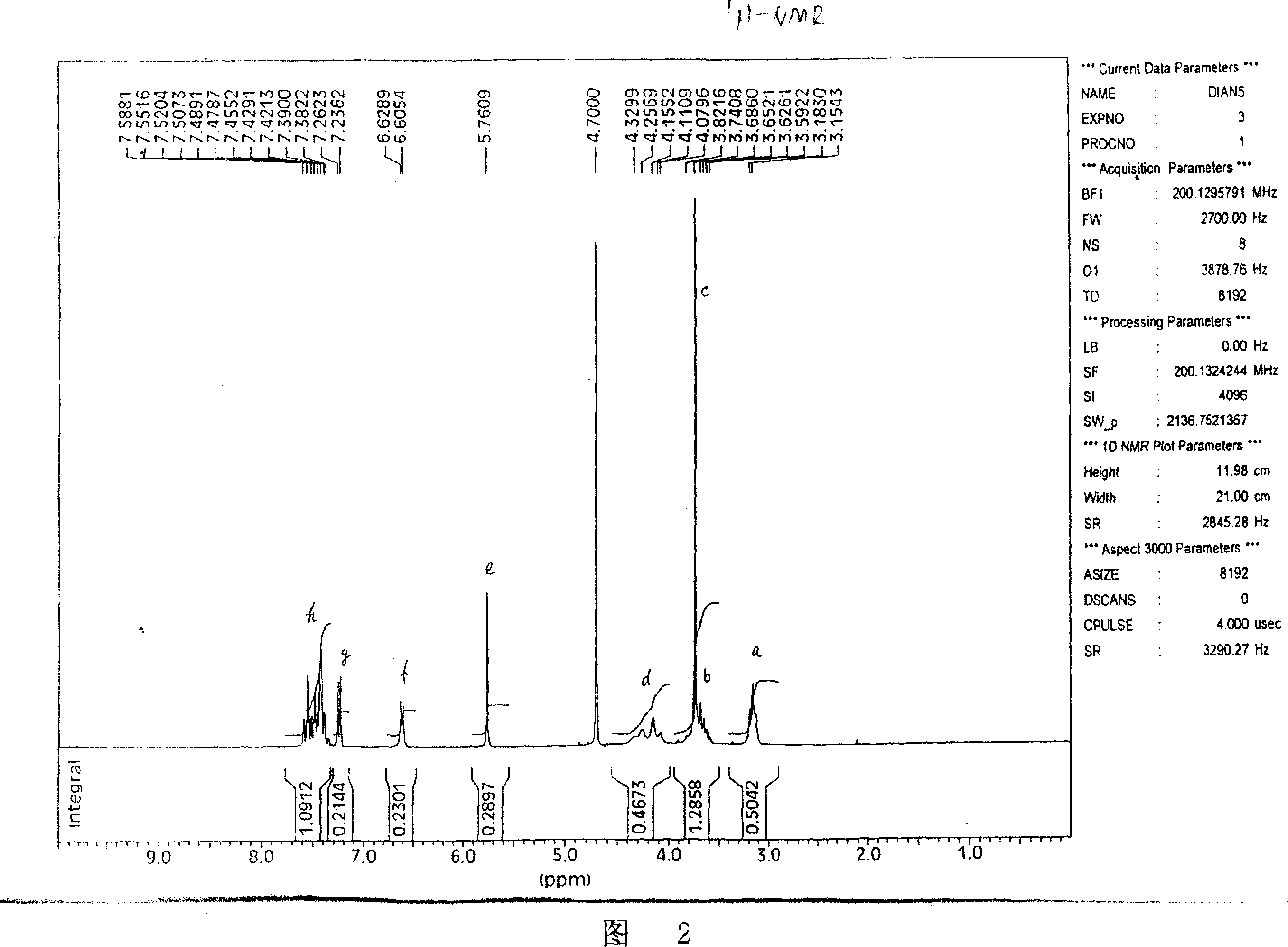
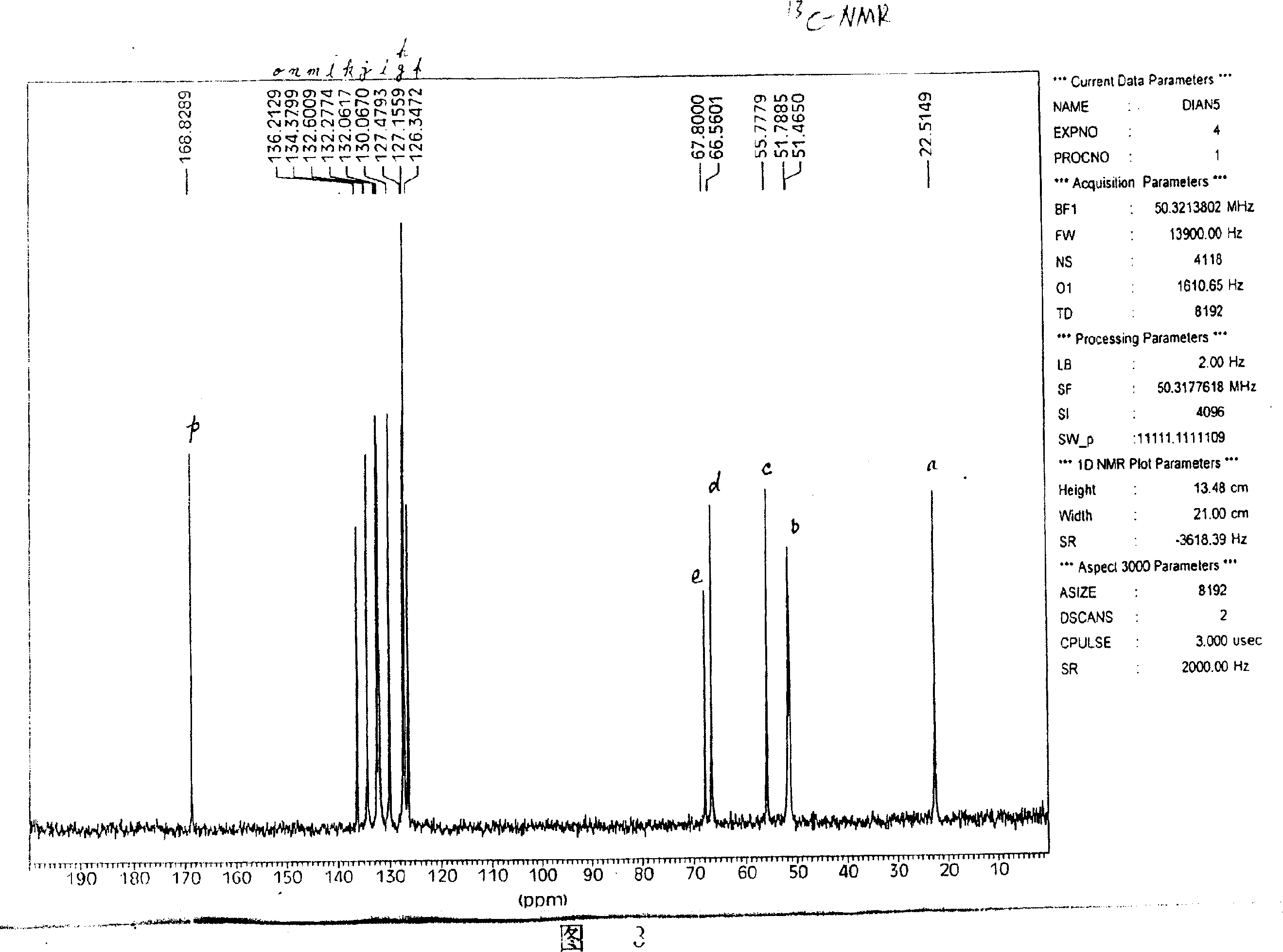
Image
Examples
Embodiment 1
[0045] Weigh 20g of racemic o-chlorophenylglycine methyl ester hydrochloride, add 50ml of water and 150ml of dichloromethane, adjust the pH to 7-8 with sodium bicarbonate while stirring, discard the water layer after liquid separation, and use the dichloromethane layer as Wash with 50ml of water three times, dry over sodium sulfate and evaporate the solvent under reduced pressure to obtain 16g of oily substance. Dissolve the oil in 200ml of isopropanol, add 9.6g of L-tartaric acid, stir at 50°C for 0.5 hours, add seed crystals when the temperature drops to 35°C naturally, stir overnight at room temperature, cool to 20°C and stir for 4 hours, then wash the solid with suction Dry to get (+) o-chlorophenylglycine methyl ester tartrate.
[0046] The separated mother liquor was evaporated to dryness at 50°C under reduced pressure to obtain 100g of oily substance. Add 200ml of water and 200ml of dichloromethane, and adjust the pH to 7-8 with sodium bicarbonate while stirring. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com