Oral formulation containing itraconazole and methods for manufacturing and using the same
A technology for itraconazole and pharmaceutical preparations, applied in the field of preparation and application of the oral pharmaceutical preparations, can solve the problems of low solubility and bioavailability, low pKa value, low solubility and the like of itraconazole and saconazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
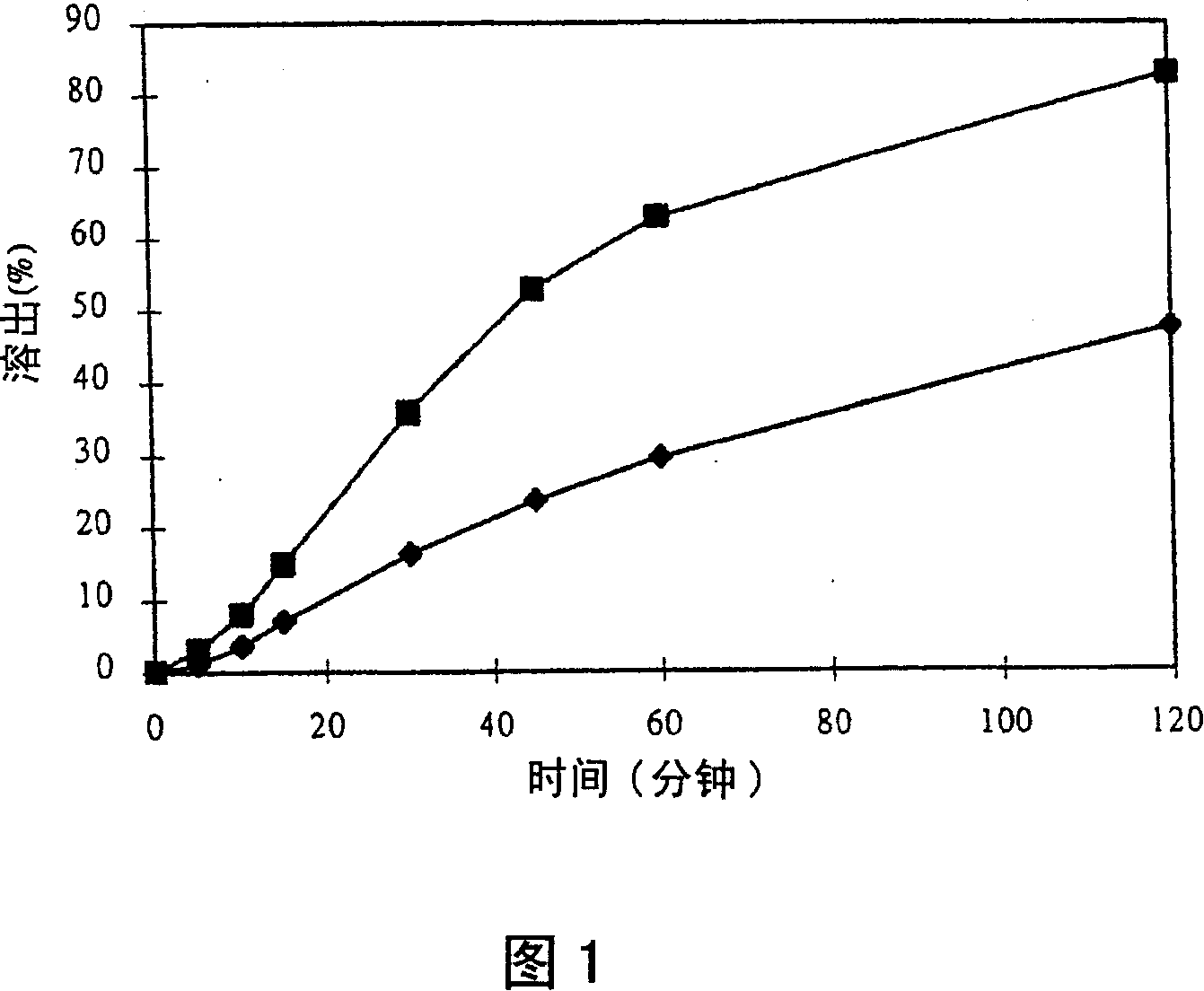
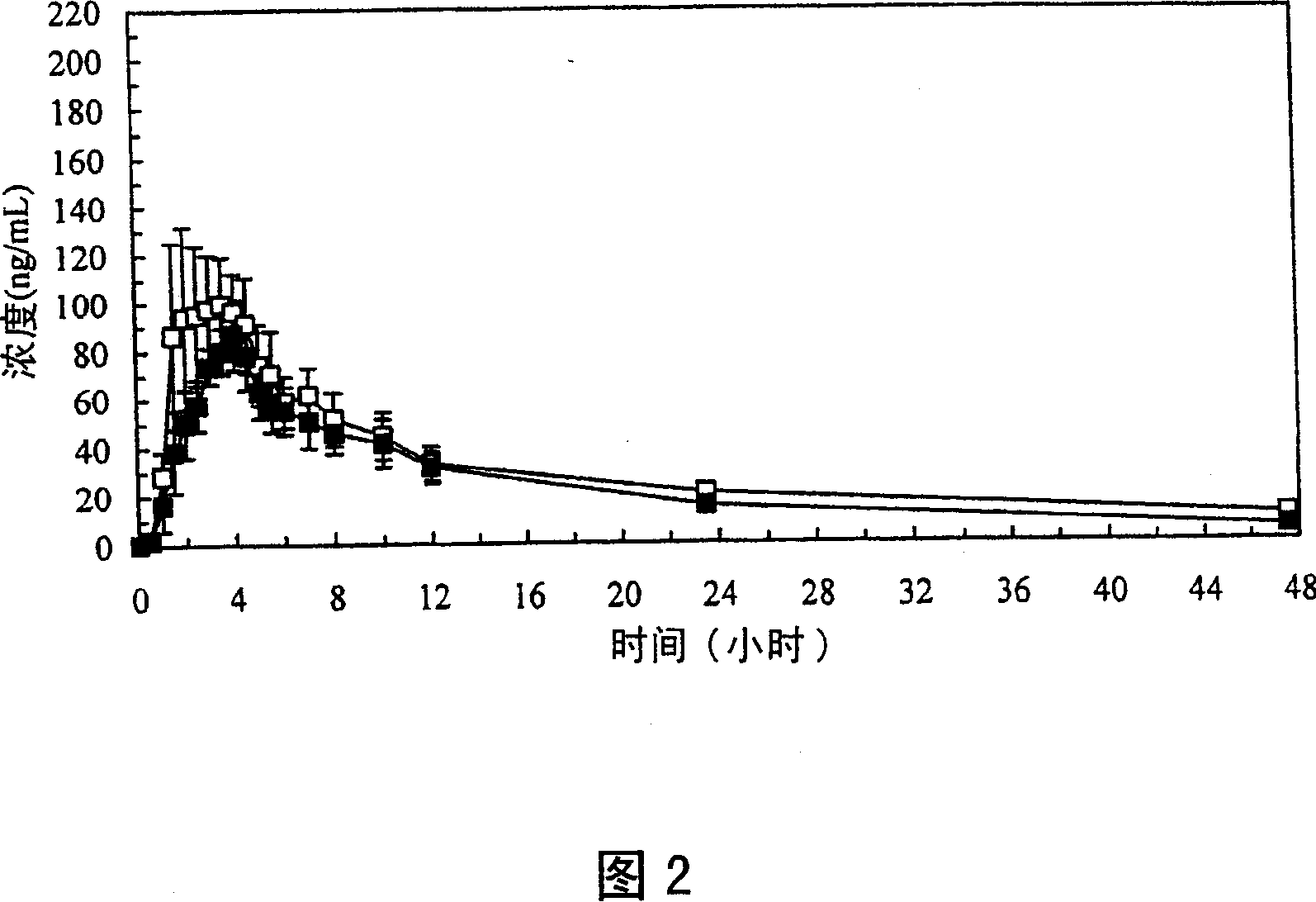
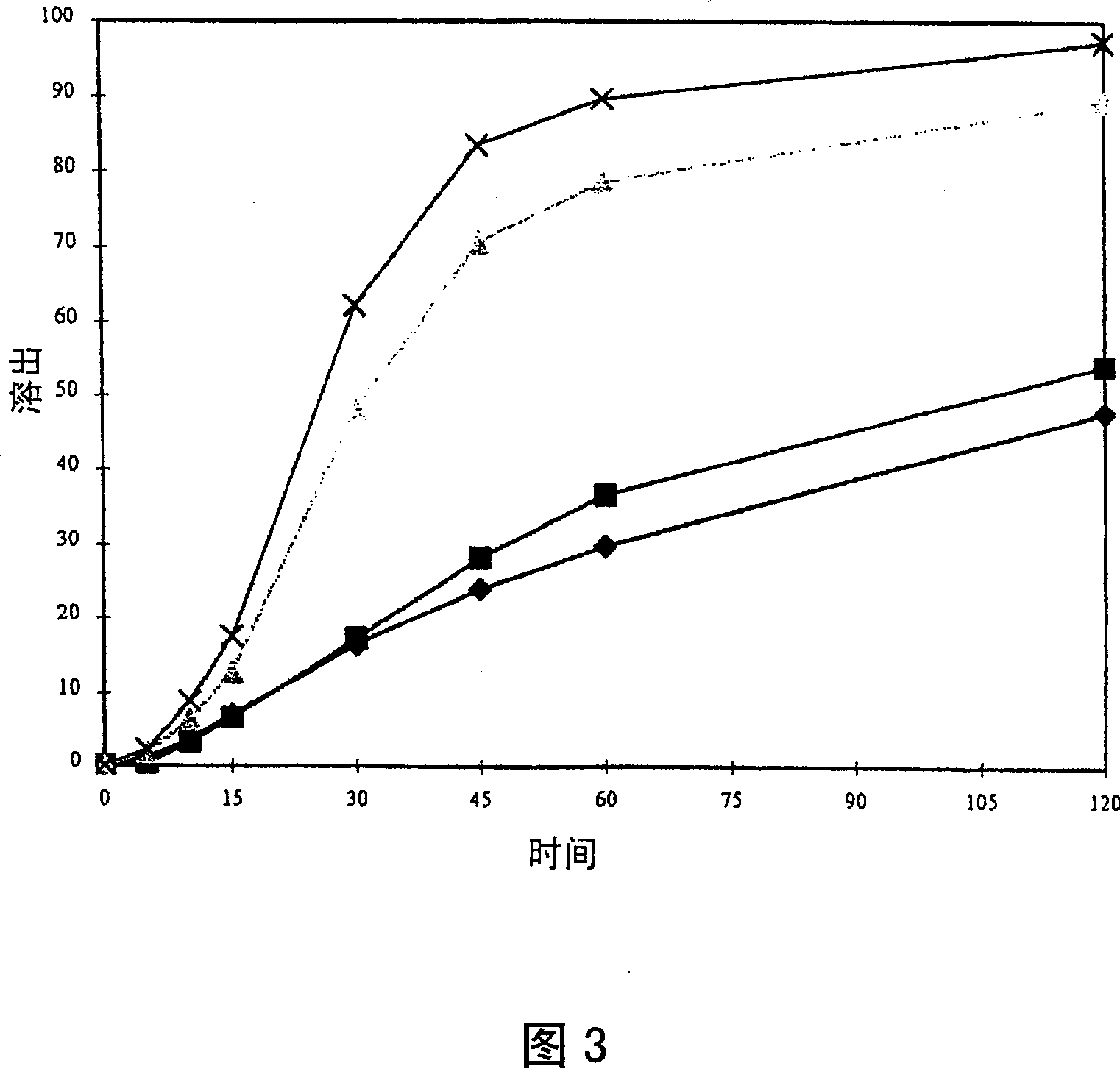
Image
Examples
Embodiment 1
[0065] (A) Materials and methods for preparing the core:
[0066] The cores were prepared using the following ingredients:
[0067] Element
Dosage
Polyvinylpyrrolidone (PVP K-30)
40g
[0068] Isopropanol
300ml
200ml
400g
800g
900g
[0069] The cores were produced by a 3-step process. The first step involved dissolving 40 g of PVP K-30 in 300 ml of isopropanol with stirring and then mixing with 200 ml of distilled water, which produced a binder solution. The second step involves mixing together 800g of starch and 900g of talc. The final step consists of adding sucrose to a fluid bed granulator (such as Glatt or Huttlin) and spraying the PVP K-30 binder solution produced in the first step onto the sucrose while the starch-talc mixture Add to sucrose to form a nucleus. The cores are further dried in warm air.
[0070] T...
Embodiment 2
[0078] The core and drug coating of the drug formulation in Example 2 were prepared according to the procedure described in Example 1, except that the amount of core used in Example 2 was different from that in Example 1. The pharmaceutical preparation of embodiment 2 contains following composition:
[0079] Element
Embodiment 3
[0081] The core and drug coating of the drug formulation of Example 3 were the same as those described in Examples 1-2, except that the drug formulation of Example 3 contained a protective layer serving as a seal coat of the drug coating. The pharmaceutical preparation of embodiment 3 contains following composition:
[0082] Element
Dosage
1. Core: 1155g
2. Drug coating:
600g
Hydroxypropyl Methyl Cellulose (HPMC)
945g
6900ml
12600ml
3. Protective layer:
Polyethylene glycol (PEG) 20000
27g
270ml
[0083] A protective layer was prepared by adding distilled water to PEG 20,000 followed by stirring until PEG 20,000 was completely dissolved.
[0084] After the drug-coated particles are made in the Glatt and dried, while the Glatt is still centrifuging, the protective layer is sprayed onto the drug-c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com