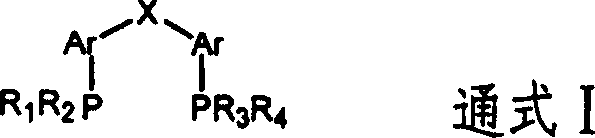Method for preparing Beta, Gamma unsaturated ester
A technology of unsaturated and conjugated olefins, applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., can solve the problem of low catalyst system efficiency, low selectivity of catalyst system, low yield of target products, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen protection, 0.05mmol palladium acetate, 0.11mmol αα'-bis(diphenylphosphino)phenyl ether (Oxydi-2,1-phenylene)bis(diphenylphosphine), 328.00mmol methanol, 0.718g n- Nonane (internal standard) and 50ml diphenyl ether or anisole, then add 286.50mmol 1,3-butadiene, and fill with carbon monoxide to a pressure of 5MPa. The reaction was stirred for 4 hours in an oil bath at 230°C. After the reaction was completed, it was cooled to room temperature, and the product was analyzed by gas chromatography-mass spectrometry. The results showed that 1,3-butadiene was completely converted, the selectivity of 3-pentenoic acid methyl ester (3-MP) was above 96%, and the gas chromatography yield was 96%.
Embodiment 2
[0031] Under nitrogen protection, in 250ml autoclave, add 0.05mmol palladium acetate, 0.4mmol αα'-bis(diphenylphosphino)phenyl ether, other reaction conditions are the same as embodiment 1. After the reaction was finished, it was cooled to room temperature, and the product was analyzed by gas chromatography-mass spectrometry, and the gas chromatography yield of methyl 3-pentenoate was 84.8%.
Embodiment 3
[0033] Under nitrogen protection, add 0.05mmol palladium acetate, 0.06mmol αα'-bis(diphenylphosphino)phenyl ether, 328.00mmol methanol, 0.072g n-nonane (internal standard) and 10ml anisole in 250ml autoclave, then Add 286.50 mmol of 1,3-butadiene, and fill with carbon monoxide to a pressure of 3 MPa. The reaction was stirred for 4 hours in an oil bath at 230°C. After the reaction was completed, it was cooled to room temperature, and the product was analyzed by gas chromatography-mass spectrometry, and the gas chromatography yield of methyl 3-pentenoate was 35.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com