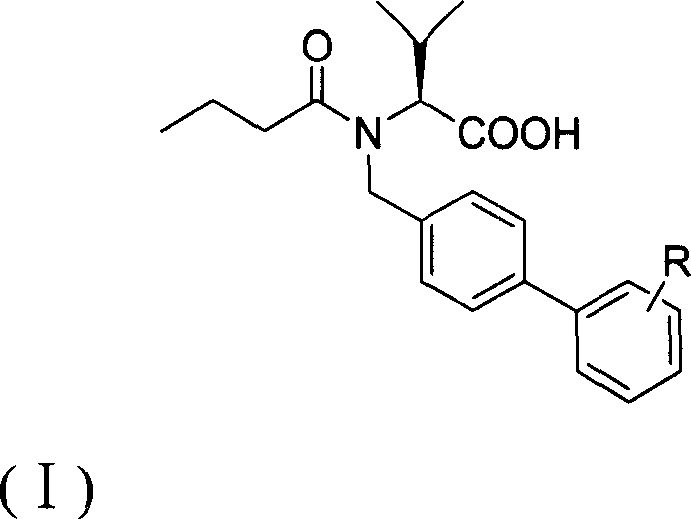
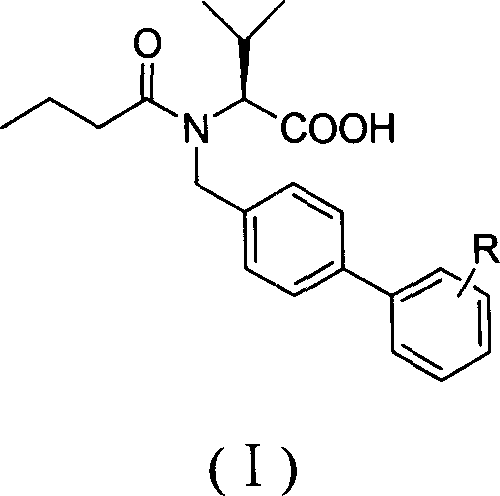
A compound butyryl biphenyl of valine
A technology for valine butyryl biphenyl and compound, which is applied in the field of medicinal chemistry and can solve the problems of easy explosion, toxicity, shortening of the existence time of the compound and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] [Example 1] (S)-N-n-butyryl-N-[4-(2-(5-oxo-1,2,4-oxadiazole-3)phenyl)]benzyl valline acid
[0018] Step 1: (L)-Valine Methyl Ester Hydrochloride
[0019] Add 5mL of anhydrous methanol to a 50mL three-necked flask under ice-bath conditions, stir, slowly add 1mL of thionyl chloride dropwise, after the dropwise addition, stir for 30min, add 1g (L)-valine, stir overnight, reduce Solvent was removed by autoclaving. Recrystallized from methanol-ether to obtain 1.35 g of a colorless needle-like solid, mp: 128-132°C.
[0020] Step 2: N-[4-(2-cyanophenyl)]benzyl-L-valine methyl ester
[0021] Under N2 protection conditions, dissolve 2.0g (L)-valine methyl ester hydrochloride in 15mL DMF, cool in an ice bath, stir, add 5mL triethylamine dropwise, and then add 3.0g 2'-cyano- 4-Bromomethylbiphenyl. React at 70°C and monitor by TLC. After the reaction is complete, cool down rapidly, add 15 mL of distilled water, and extract with ethyl acetate. Combined organic phases, KHCO 3 ...
Embodiment 2
[0033] [Example 2] Antihypertensive drug activity screening experiment
[0034] Experimental animals: 30 spontaneously hypertensive rats (SHR), healthy, half ♀♂ (female not pregnant), purchased from Shanghai Bikai Experimental Animal Co., Ltd., certificate number: Shanghai Dynamic Hezheng Zi No. 152 ;
[0035] Drug under test: antihypertensive active compound (S)-N-n-butyryl-N-[4-(2-(5-oxo-1,2,4-oxadiazole-3)phenyl)]benzo valine (compound 1).
[0036] Positive control drug: losartan, the clinical dosage is 50mg / kg, assuming that the body weight is 60kg, the human dosage is 5 / 6mg / kg, converted to a rat dosage of 5mg / kg, the molecular weight of losartan is 461, converted into moles The concentration is 1.86mol / L, and the positive control drug is equivalent to the dose in the efficacy experiment (the ratio of low, medium and high doses is 1:2:4), which is set at 3.72mol / L.
[0037] Experimental method: 30 spontaneously hypertensive rats (SHR) models were selected and divided i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com