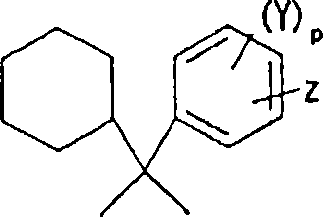
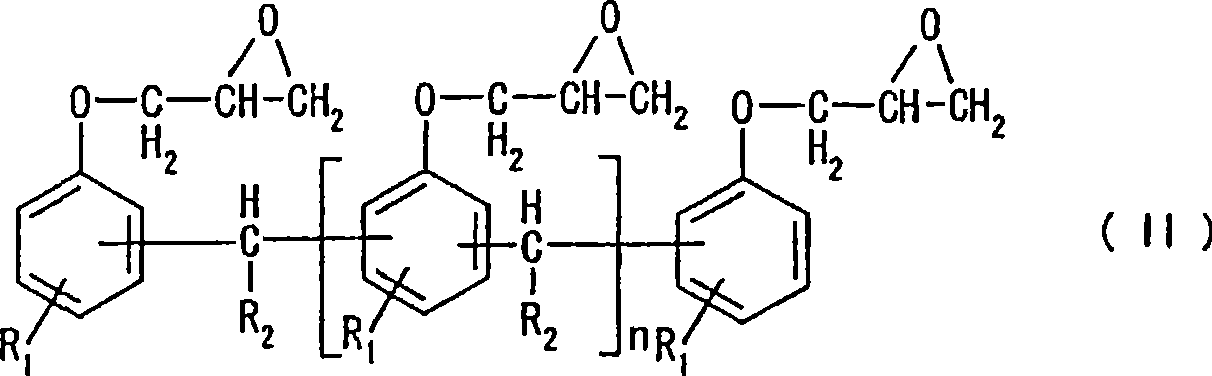
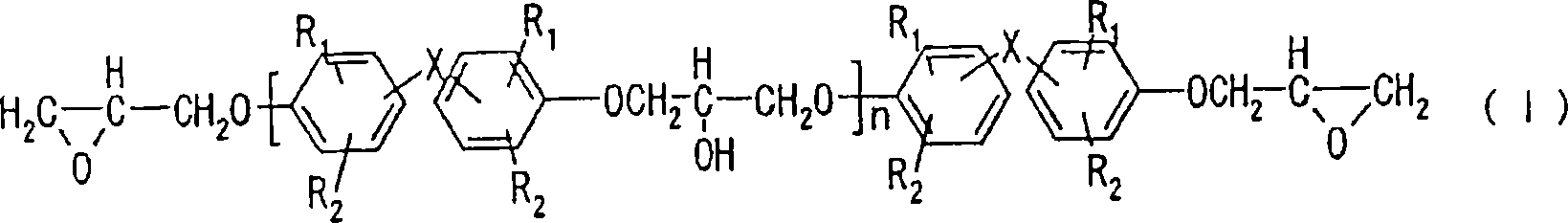Alkali development-type photosensitive resin composition, substrate with protrusions for liquid crystal split orientational control and color filter formed using the same, and liquid crystal display d
A technology of photosensitive resin and composition, applied in nonlinear optics, photoengraving process of pattern surface, photosensitive material used in opto-mechanical equipment, etc., can solve problems such as insufficient sensitivity, difficulty in obtaining pattern shape and fine pattern, etc. , to achieve the effect of excellent sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0181] Hereinafter, the present invention will be described in further detail by citing examples and the like, but the present invention is not limited to these examples. Also, in the following Examples and the like, "%" indicates mass%.
[0182]In manufacture examples 1-7 and comparative manufacture examples 1-3, the alkali developable resin composition containing the photopolymerizable unsaturated compound as binder resin was manufactured. In Examples 1 to 7 and Comparative Examples 1 to 3, an alkali developing type photosensitive resin composition was produced by mixing a solvent and a photopolymerization initiator in these alkali developable resin compositions, and in Examples 8 to 14 and In Comparative Examples 4-6, the coloring alkali image development type photosensitive resin composition was manufactured by further mixing a coloring agent.
[0183] Commercially available items used as the polyfunctional epoxy resin (A) in the following examples are as follows.
[018...
manufacture example 1
[0189] [Manufacture Example 1] Manufacture of Alkaline Developable Resin Composition No.1
[0190] Add 154g Adeka Resin EP-4100E (manufactured by Asahi Denka Industry Co., Ltd.; bisphenol A type epoxy resin, epoxy equivalent is 190; hereinafter also referred to as compound a-1), 55.2g YP-90LL (manufactured by Yasuhara Chemical Co., Ltd.; ring The content of terpene monophenol is 90%; the average molecular weight is 266, and the hydroxyl equivalent is 340; hereinafter also referred to as compound c) and 90.3g propylene glycol monomethyl ether acetate, and the temperature is raised to 115°C. 1.05 g of triphenylphosphine was slowly added, followed by stirring at 120° C. for 4 hours. Furthermore, 194 g of propylene glycol monomethyl ether acetates were added, and it cooled to 50 degreeC or less. Thereafter, 0.26 g of 2,6-di-tert-butyl-p-cresol, 2.6 g of benzyltriethylammonium chloride, and 46.8 g of acrylic acid (hereinafter, also referred to as compound b) were added, and the te...
manufacture example 2
[0192] [Manufacture Example 2] Manufacture of Alkaline Developable Resin Composition No. 2
[0193] Add 171g of Adeka Resin EP-4100E (epoxy equivalent of 190; compound a-1), 25g of Epicoat 834 (manufactured by Nippon Epoxy Resin Co., Ltd.: epoxy equivalent of 250; hereinafter also referred to as compound a-2) and 187g of YP -90LL (Compound c), the temperature was raised to 115°C. 1.15 g of triphenylphosphine was slowly added, followed by stirring at 120° C. for 4 hours. Furthermore, 617 g of propylene glycol monomethyl ether acetates were added, and it cooled to 50 degreeC or less. Thereafter, 0.415 g of 2,6-di-tert-butyl-p-cresol, 4.15 g of benzyltriethylammonium chloride, and 32.4 g of acrylic acid (compound b) were added, and the temperature was raised to 120° C. and kept for 5 hours. After cooling to 50° C. or lower, 88.2 g of diphthalic dianhydride (compound d-1) and 0.289 g of tetrabutylammonium bromide were added, and the temperature was raised to 120° C., and kept fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com