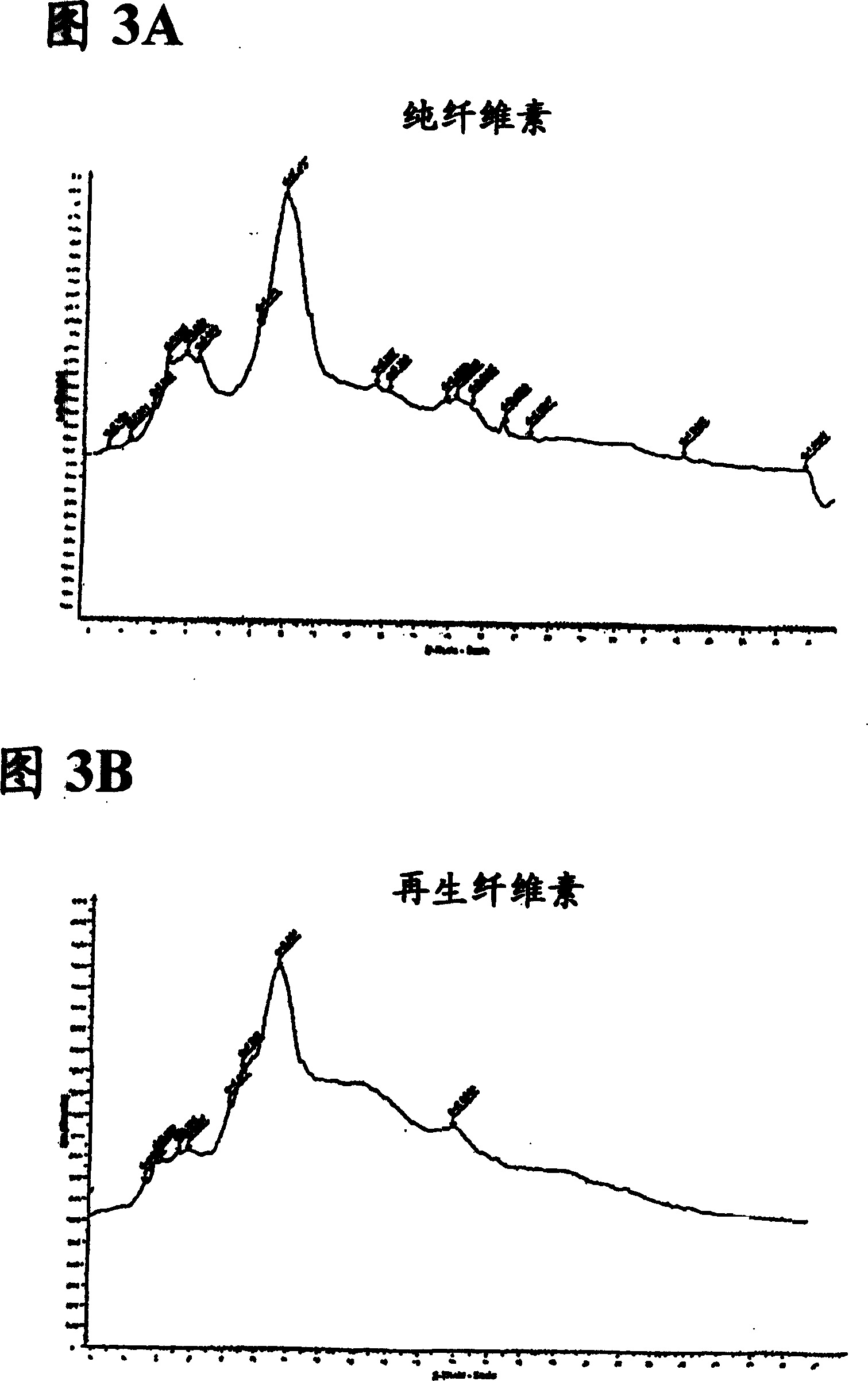
Dissolution and processing of cellulose using ionic liquids
A technology for dissolving cellulose and ionic liquid, applied in the field of dissolving cellulose, can solve the problems of no practical value and unacceptable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Cellulose Dissolving
[0100] We found that the best ionic liquid for dissolving an exemplary test material, regenerated fibrous cellulose, was [C 4 mim] Cl. In the general method, fibrous cellulose (0.2 g) was placed in a glass vial of molten [C 4 mim]Cl (2 g) and heated in a domestic microwave oven with 3 x 5 second heating pulses. After each heat pulse, the vial was removed and vortexed to mix the contents before being placed back in the microwave. Optically clear viscous solutions of cellulose in ionic liquids were obtained.
[0101] Solutions of cellulose dissolved in ionic liquids at different concentrations can be prepared in this way. The viscosity of the solution increases with the increase of cellulose concentration. When the cellulose concentration reaches 25% by weight, a transparent solution can still be obtained. When the cellulose concentration is higher, an opaque viscous gel is formed. For cellulose in [C 4 The actual solubility lim...
Embodiment 2
[0104] Example 2: Dissolution of cellulose in 1,3-dialkylimidazolium salts with anions and cations functional relationship
[0105] Compared with conventional solvents, cellulose is more easily dissolved in ionic liquids at high concentrations. Screening of ionic liquids with different cations as their chloride salts. These salts include [C 6 mim]Cl and [C 8mim] Cl. It was found that the solubility of cellulose in imidazolium-based ionic liquids decreased with increasing cationic alkyl chain length.
[0106] Screens for various anions, from small hydrogen bond acceptors (Cl - ) to large noncoordinating anions (tetrafluoroborate and hexafluorophosphate) as [C 4 mim] + Salt. Anions include Cl, Br, thiocyanate, perchlorate, hexafluorophosphate and tetrafluoroborate. These results are shown in Table 1 below.
[0107] We found that ionic liquids containing anions (halogens and pseudohalogens) with strong hydrogen bond acceptors can obtain good dissolution effects. All...
Embodiment 3
[0116] Example 3: Cellulose regeneration
[0117] We have found that cellulose can be precipitated from ionic liquids by adding water. This incompatibility is the basis for the regeneration method described below.
[0118] By adding a known amount of water to the ionic liquid, then using microwave heating to carry out the dissolution process to determine the water that can still maintain the solvent properties of the ionic liquid in [C 4 mim] concentration in Cl. When the moisture content of the ionic liquid is greater than about 1% by weight (about 0.5 mole fraction of H 2 O), the solvent properties were significantly weakened, and we found that the fibrous cellulose was no longer soluble.
[0119] When a high concentration of cellulose (greater than 10% by weight) is dissolved in [C 4 mim]Cl, a solution with an optical anisotropy between that of a crossed polarizing filter and one exhibiting birefringence is obtained. The formation of liquid crystal solutions of cellu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com