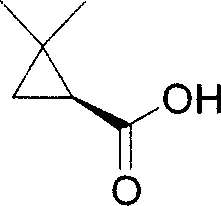
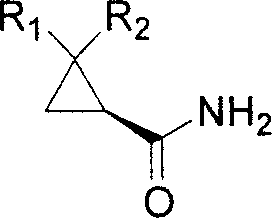
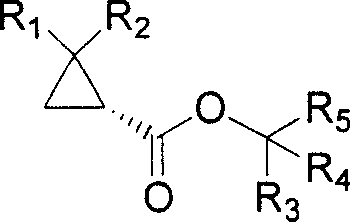
Optically active cyclopropanecarboxamide preparation method
A technology for cyclopropanecarboxamide and optically active substances is applied in the field of preparing cyclopropanecarboxamide optically active substances, and can solve the problems of enzymatic agglomeration, long reaction process, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0072] Preparation of 2,2,2-trifluoroethyl cyclopropanecarboxylate
[0073]
[0074] (S)-Dimethylcyclopropanecarboxylic acid (R)-Dimethylcyclopropanecarboxylic acid
[0075] -2,2,2-trifluoroethyl ester (50%) -2,2,2-trifluoroethyl ester (50%)
[0076] At normal temperature, add 500ml of dichloromethane and 228g of dimethylcyclopropanecarboxylic acid into the reactor, and stir evenly. Under the condition of keeping 20°C, thionyl chloride (SOCl 2 ) 236g. After stirring at room temperature for 1 hour, 190 g of 2,2,2-trifluoroethanol (2,2,2-trifluoroethanol) was slowly added dropwise, followed by stirring at room temperature for 2 hours. with saturated sodium carbonate (Na 2 CO 3 ) solution and washed with anhydrous sodium sulfate (Na 2 SO 4 ) drying and distillation under reduced pressure to remove the organic solvent to obtain dimethylcyclopropanecarboxylic acid-2,2,2-trifluoroethyl ester racemic mixture 340g (87% yield, (S) and (R) configuration each 50 %).
preparation example 2-5
[0078] Preparation of Racemic Mixture of Dimethylcyclopropane Carboxylate
[0079]
[0080] (S)-Dimethylcyclopropanecarboxylate (R)-Dimethylcyclopropanecarboxylate
preparation example
[0081] Each preparation example is distinguished according to the alcohol used: 2,2,2-trichloroethanol (preparation example 2), 2-methoxyethanol (preparation example 3), 2-bromoethanol (preparation example 4), 2-fluoroethanol Ethanol (preparation example 5). Various racemic dimethylcyclopropane carboxylates were prepared according to the procedure described in Preparation 1. The results are shown in Table 1.
[0082] Table 1
[0083] Preparation Example No.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com