Pharmaceutical in vivo dynamics characteristic-nondestructive in situ monitoring system and monitoring method
A monitoring system and drug technology, applied in pharmaceutical formulations, pharmaceutical sciences, preparations for in vivo experiments, etc., to achieve the effect of reducing background interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
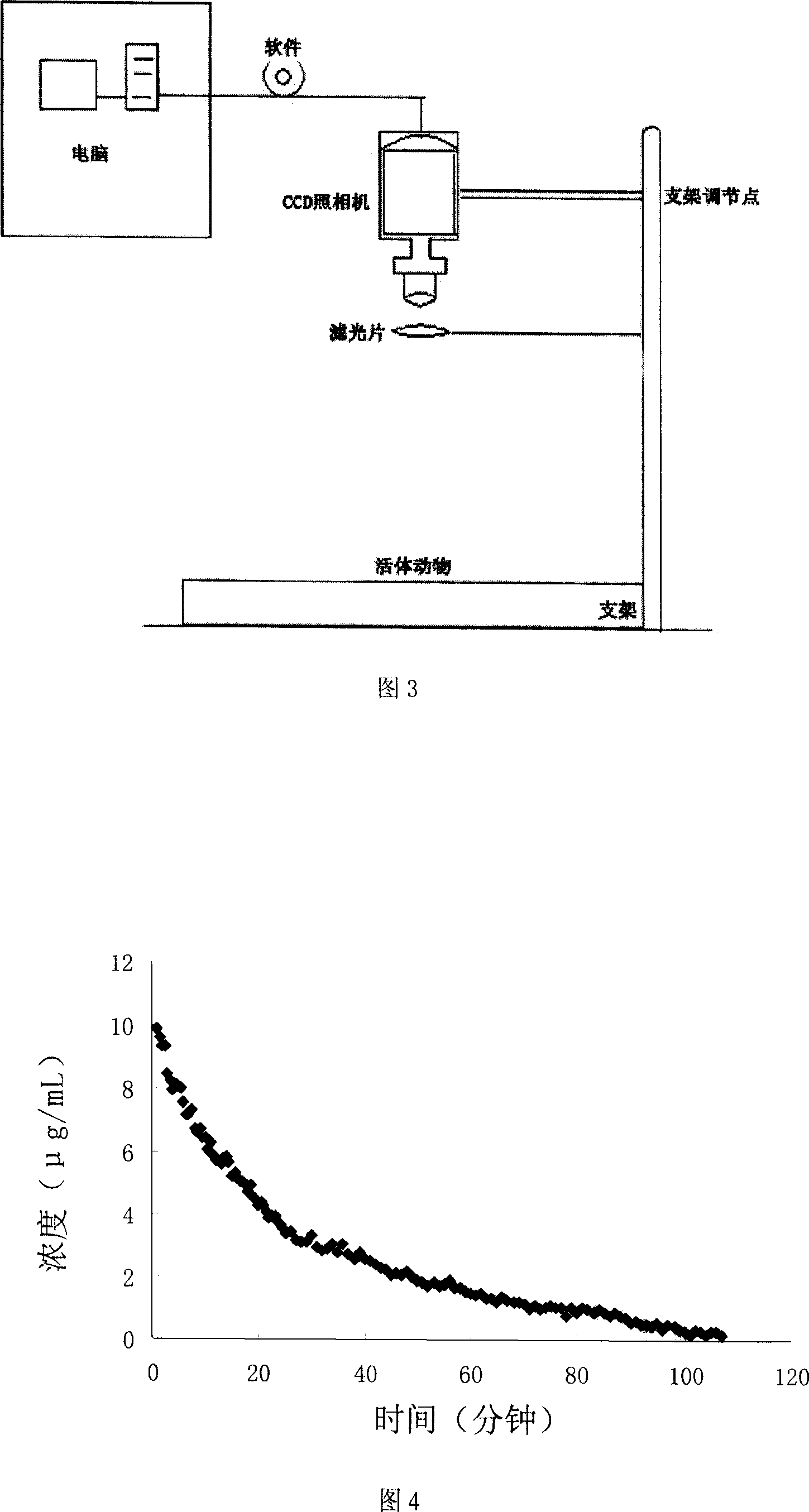
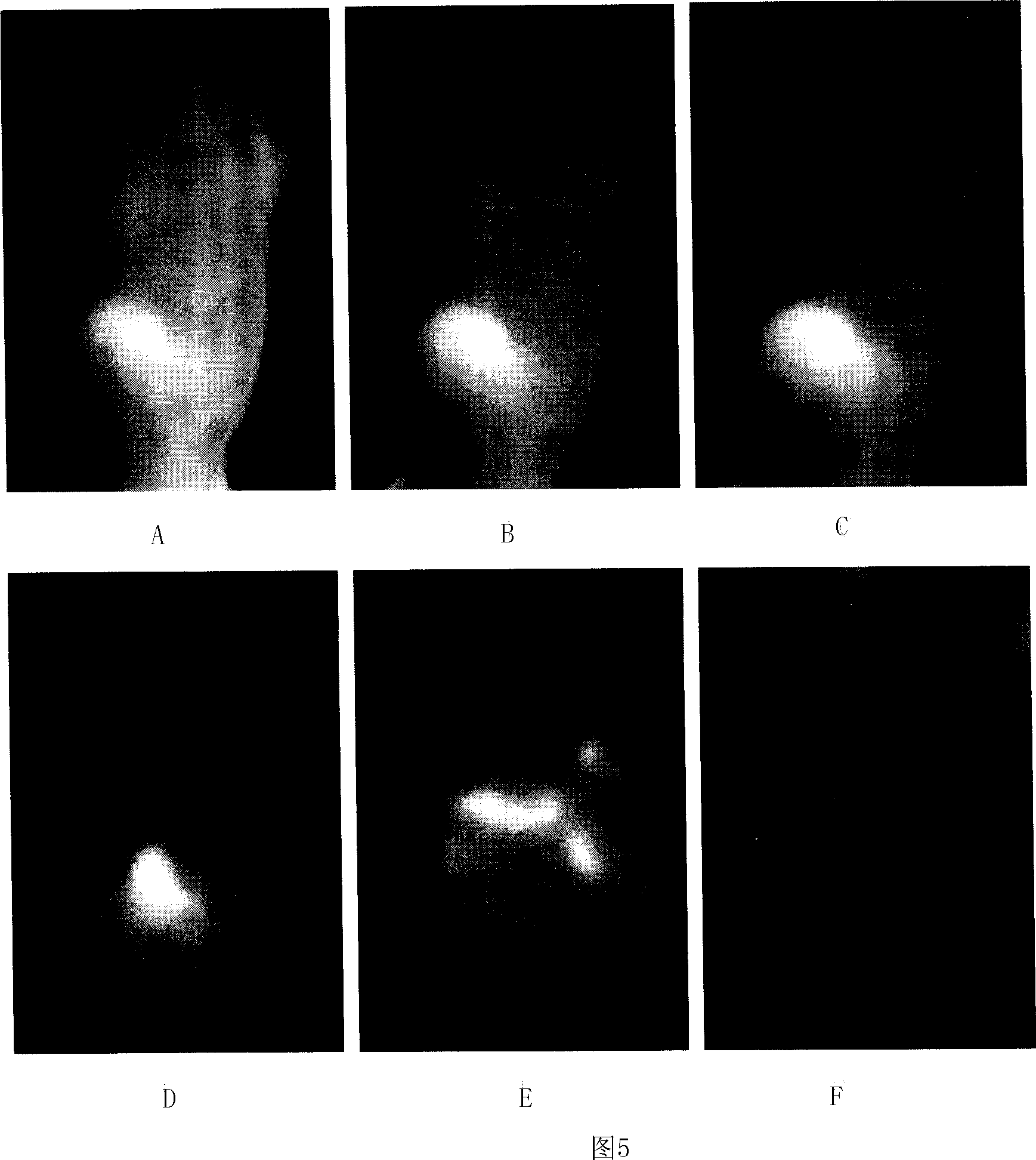
Image
Examples
Embodiment 1
[0043] Synthetic method of cypate-labeled insulin
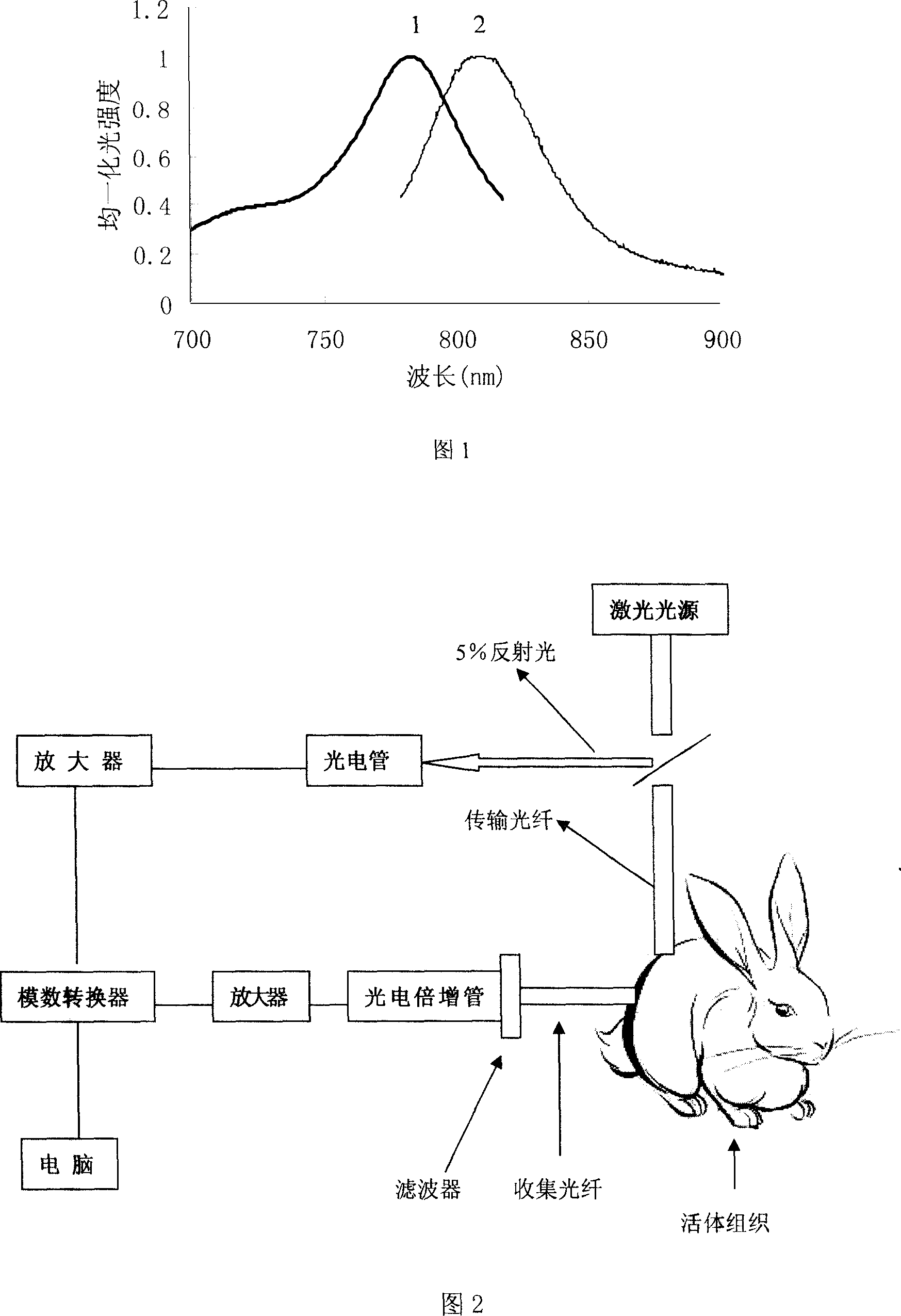
[0044] Dissolve 1mg cypate (see Figure 1 for the spectrum) in anhydrous DMSO, add 0.4mgHBTU and 0.3mgHOBT to it, vortex and mix well in the dark, then slowly add 4ml 1.25mg / ml of the activation solution dropwise while shaking Insulin solution (pH8.5), stirred and reacted at 4 degrees for 6 hours. After the reaction solution was mixed, it was dialyzed in PBS buffer solution (pH7.4) for two days, and the dialysate was changed every 4 hours. The resulting solution was lyophilized and stored in a -20°C refrigerator. The calculated labeling ratio of cypate to insulin was 0.57:1.
Embodiment 2
[0046] Synthetic method of cypate-labeled lysozyme
[0047] 1 mg of cypate was dissolved in anhydrous DMSO, 0.4 mg of DCC was added thereto, after vortex mixing, the reaction was shaken for 30 minutes. Add 0.3 mg HOBT into the solution, stir for 2 hours, and centrifuge to remove the supernatant to obtain the dye activation solution. The activation solution was slowly dropped into 4ml of 1.25mg / ml lysozyme solution (pH9.2) while shaking, and stirred and reacted at 4°C for 6 hours. After the reaction solution was mixed, it was dialyzed in PBS buffer solution (pH7.4) for two days, and the dialysate was changed every 4 hours. The resulting solution was lyophilized and stored in a -20°C refrigerator. The calculated labeling ratio of dye cypate to lysozyme was 1.94:1.
Embodiment 3
[0049] Synthetic method of cypate-marked recombinant L-asparaginase
[0050] Dissolve 1 mg of cypate in anhydrous DMSO, add 0.4 mg of HBTU and 0.3 mg of HOBT to it, vortex and mix well in the dark, then slowly add 4 ml of 1.25 mg / ml L-asparagine dropwise to the activation solution while shaking In the enzyme solution (pH8.5), the reaction was stirred at 4 degrees for 6 hours. After the reaction solution was mixed, it was dialyzed in PBS buffer solution (pH7.4) for two days, and the dialysate was changed every 4 hours. The resulting solution was lyophilized and stored in a -20°C refrigerator. The labeling ratio of cypate to asparaginase was calculated to be 33:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com