Cyclophosphaxene compound as flame-proof material and synthetic method thereof
A cyclophosphazene compound technology, applied in the field of cyclophosphazene compounds and their synthesis, can solve the problems of harsh synthesis conditions, less curing methods, unstable performance, etc., achieve short curing time, avoid separation and purification methods, and flame retardant effect obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
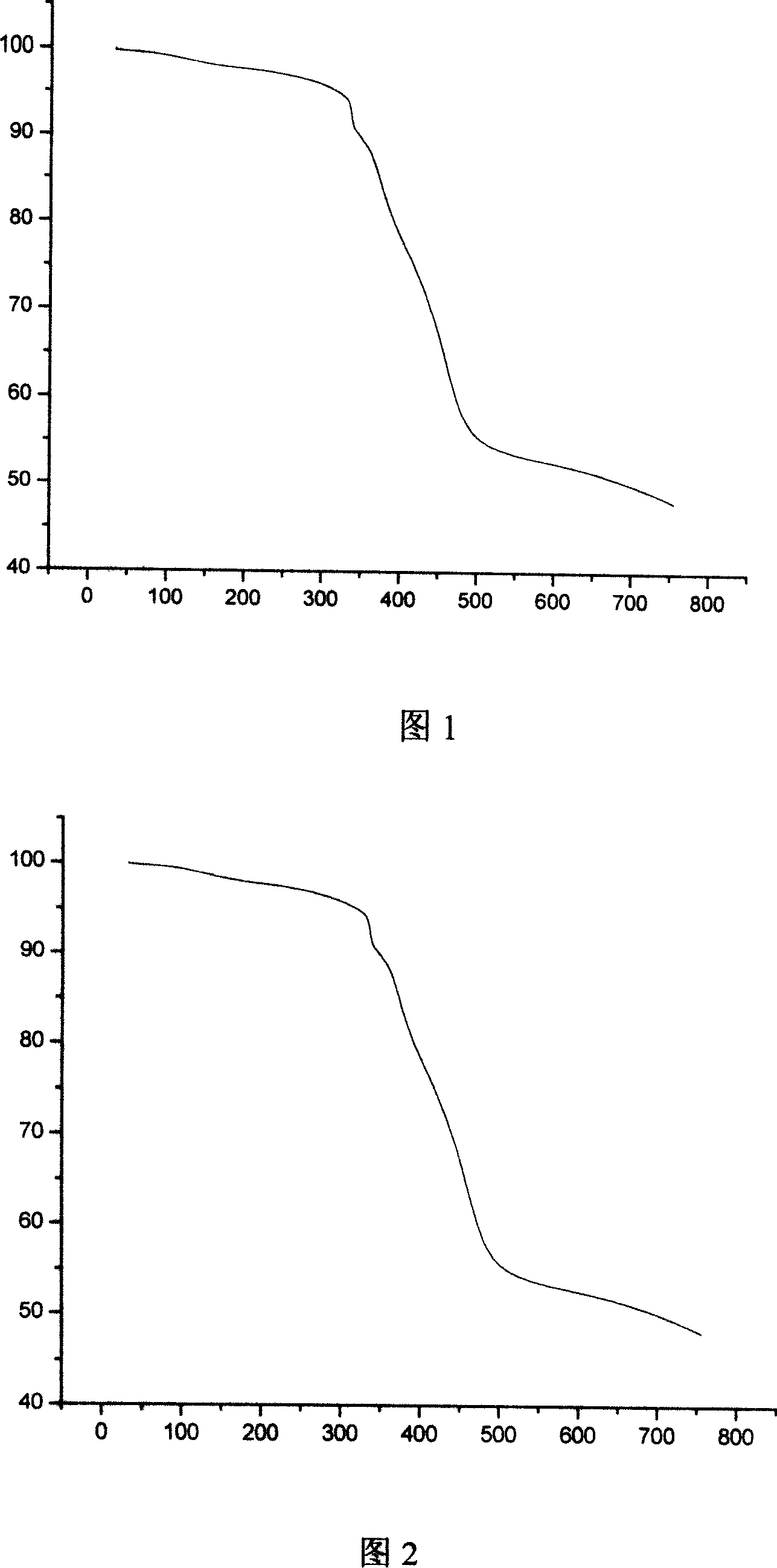
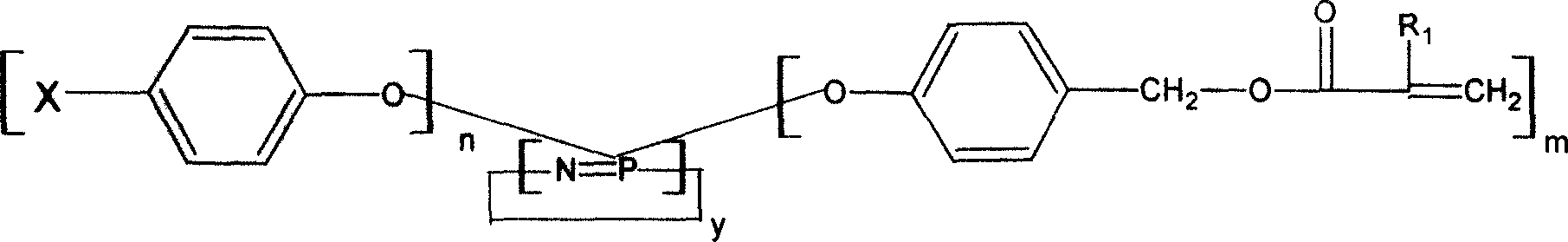
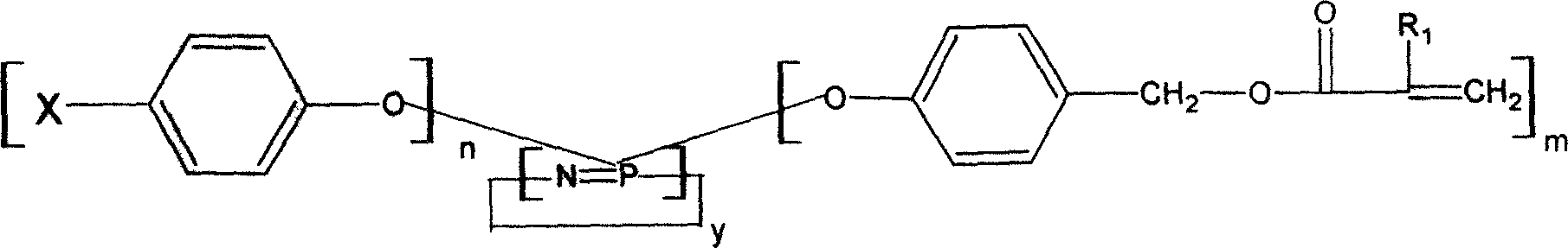
Image
Examples
Embodiment 1
[0029] Add THF 350ml, N 3 P 3 Cl 6 10g, HO-C 6 h 4 -p-CHO 25g, dry K 2 CO 3 35g, heated under reflux and stirred for 48h. The solvent was distilled off, the remaining solid was washed 3 times with distilled water, recrystallized from ethyl acetate, and dried to obtain N 3 P3 (OC 6 h 4 -p-CHO) 6 18.50g, yield 75%;
[0030] Add 600ml volume ratio THF:CH to the three-necked flask 3 OH is 1:1 mixed solvent, 10g N 3 P 3 (OC 6 h 4 -p-CHO) 6 , After dissolving, add 4g NaBH 4 , Stir at room temperature for 17h. The solvent was distilled off, recrystallized from ethanol, and dried to obtain N 3 P 3 (OC 6 h 4 -p-CH 2 Oh) 6 7g, yield 70%;
[0031] Add N to the three-neck flask 3 P 3 (OC 6 h 4 -p-CH 2 Oh) 6 -5g, 40% HBr solution 25ml, concentrated H 2 SO 4 5ml, stirred at reflux for 6h, filtered to take the solid, washed the solid with plenty of water, and dried.
[0032] Recrystallize with acetone:petroleum ether mixed solvent with a volume ratio of ...
Embodiment 2
[0035] Add 150ml THF and 7.50g PhOH to the three-necked flask, after dissolving, add 2.00gNaH, stir at room temperature for 4h, then add dropwise 80ml of 5gN 3 P 3 Cl 6 THF solution, continue to stir at room temperature for 24h after the addition is complete, add dropwise 30ml of 2.5g HOC dissolved in the mixture 6 h 4 -p-CHO and 1gNaH THF solution, reflux and stir for 20h. Filtrate, get the filtrate, distill the filtrate under reduced pressure and concentrate, dry to obtain N 3 P 3 (OPh) 5 (OC 6 h 4 CHO) 6.97g, yield 70%;
[0036] Add 60ml THF:CH to a 100ml flask 3 Mixed solvent with OH volume ratio of 9:1, 6g N 3 P 3 (OPh) 5 (OC 6 h 4 CHO), add 0.5g NaBH after dissolving 4 , stirred at room temperature for 24h. Add this mixture dropwise to dilute hydrochloric acid, collect the precipitated white solid, wash with ethanol until neutral, recrystallize from ethanol, and dry to obtain white needle-like crystals N 3 P 3 (OPh) 5 (OC 6 h 4 CH 2 OH) 4.81g, yield...
Embodiment 3
[0039] Add THF 350ml, N 3 P 3 Cl 6 10g, HO-C 6 h 4 -p-CHO 25g, dry K 2 CO 3 35g, heated under reflux and stirred for 48h. The solvent was distilled off, the remaining solid was washed 3 times with distilled water, recrystallized from ethyl acetate, and dried to obtain N 3 P 3 (OC 6 h 4 -p-CHO) 6 18.50g, yield 75%;
[0040] Add 600ml volume ratio THF:CH to the three-necked flask 3 OH is 1:1 mixed solvent, 10g N 3 P 3 (OC 6 h 4 -p-CHO) 6 , After dissolving, add 4g NaBH 4 , Stir at room temperature for 17h. The solvent was distilled off, recrystallized from ethanol, and dried to obtain N 3 P 3 (OC 6 h 4 -p-CH 2 Oh) 6 7g, yield 70%;
[0041] Add N into the three-neck flask 3 P 3 (OC 6 h 4 -p-CH 2 Oh) 6 5g, 25ml of 40% HBr solution, concentrated H 2 SO 4 5ml, stirred under reflux for 6h, filtered to take the solid, washed the solid with a large amount of distilled water, and dried. Recrystallize with acetone:petroleum ether mixed solvent with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com