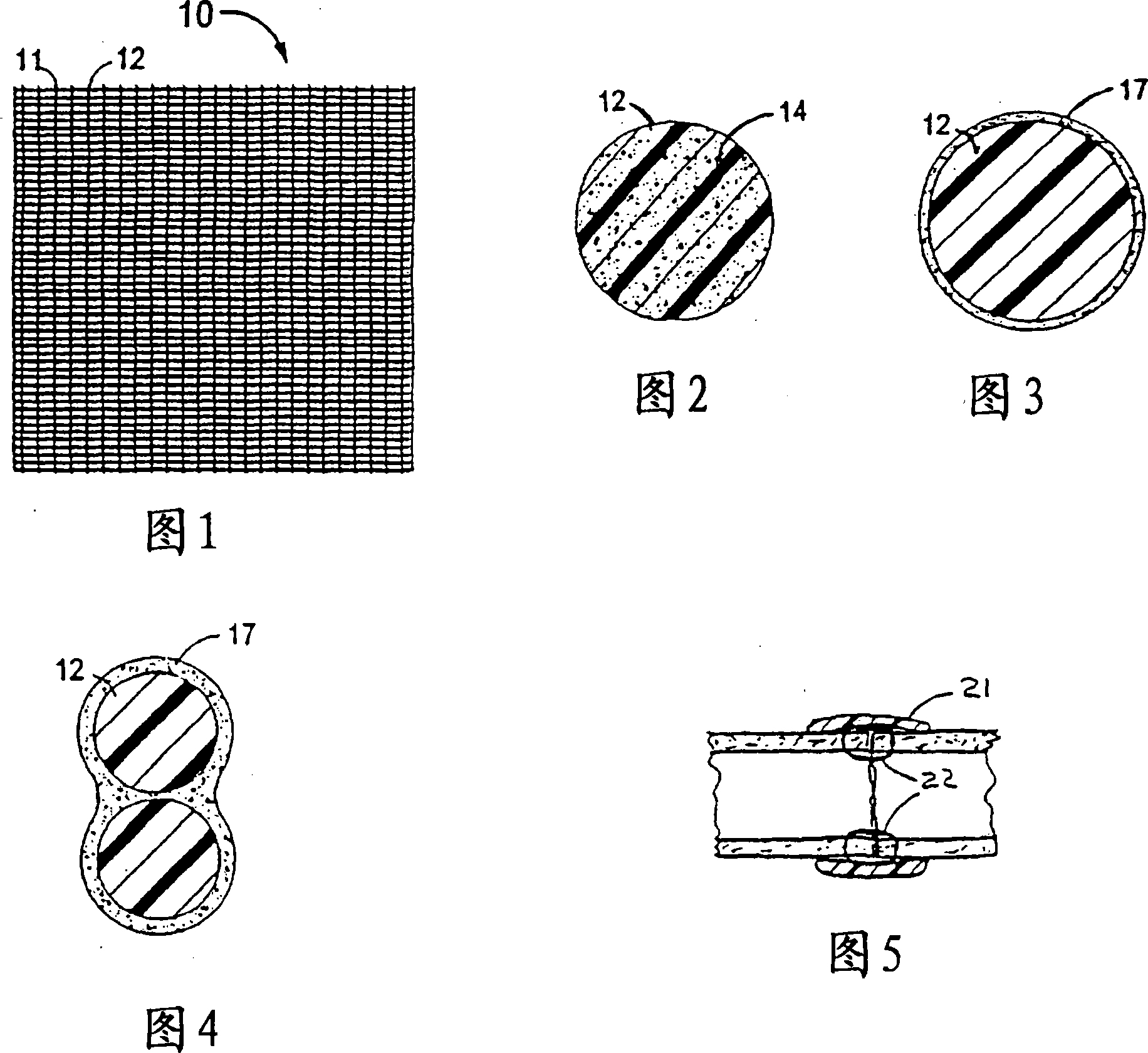
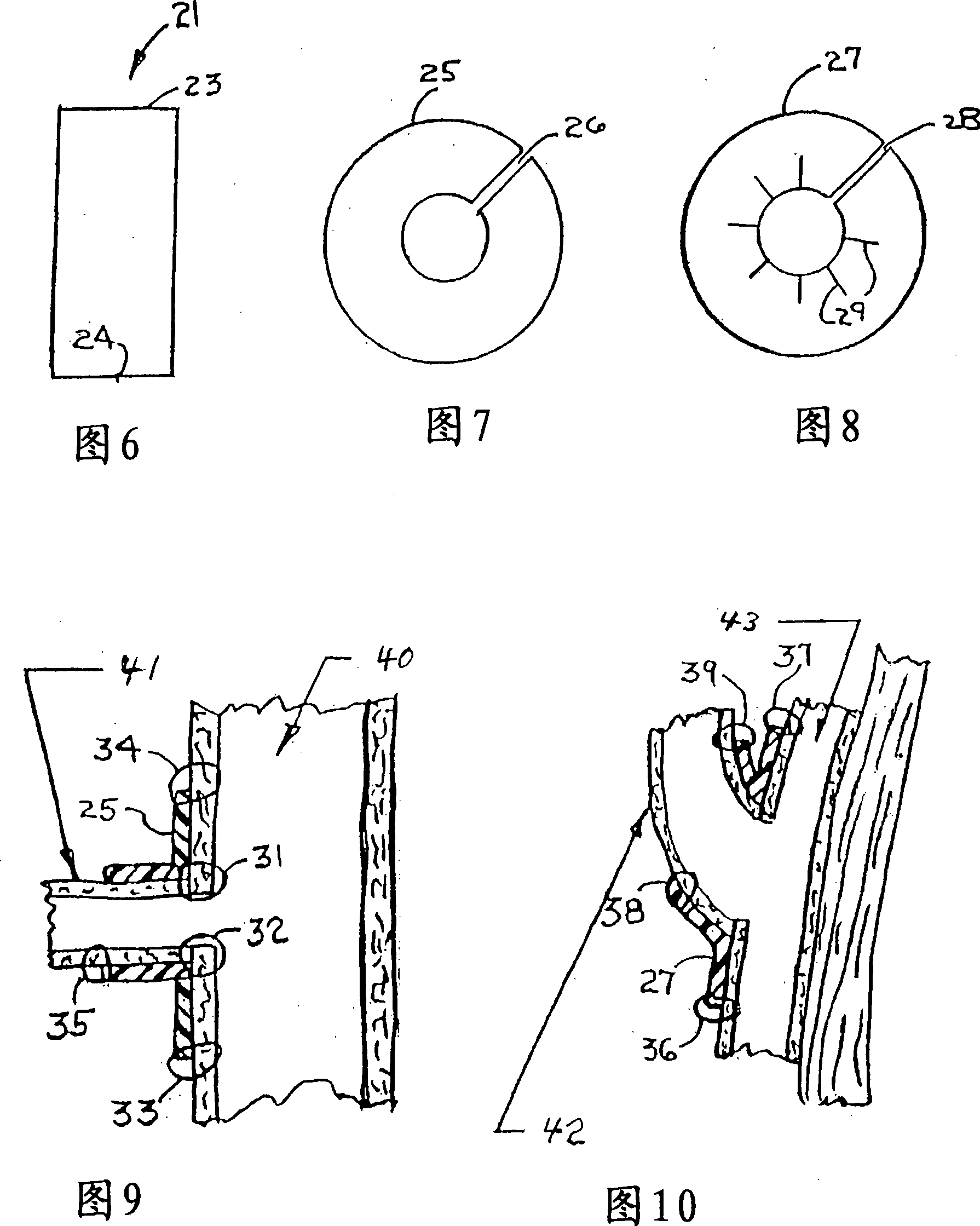
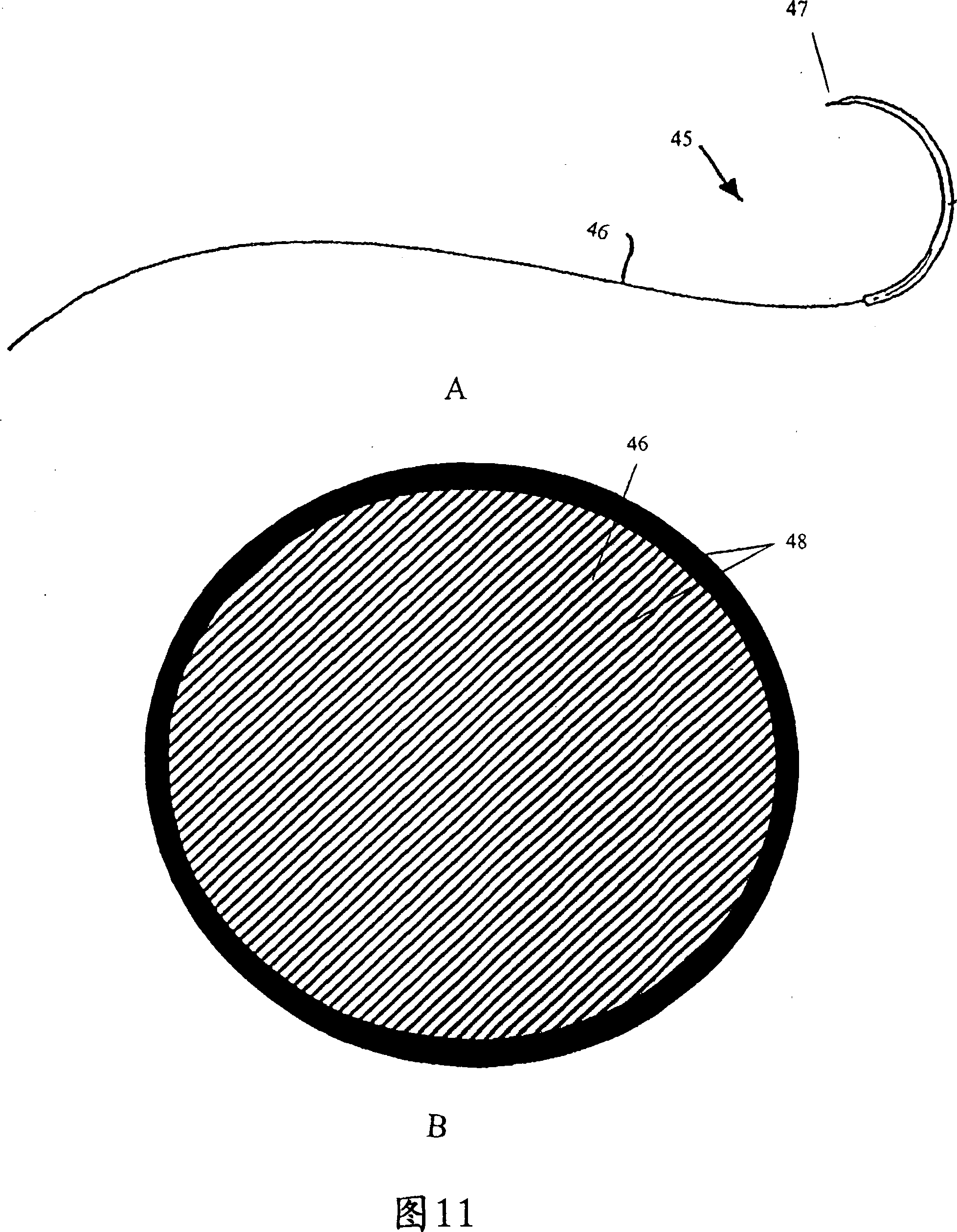
Combination drug therapy for reducing scar tissue formation
A composition and polymer technology, applied in the field of anti-platelet drug devices, medical devices for preventing scar tissue and/or adhesion formation, and anti-proliferative drug devices, which can solve the problem of low medical risk and high therapeutic benefit And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0308] Study on Prevention of Pericardial Adhesion in Rabbits
[0309] This example predicts that one embodiment of a hydrogel-based bioadhesive comprising sirolimus and analogs of sirolimus, gemmilofiban, and bivalirudin is effective in preventing postoperative adhesions and scarring. ability in. A standardized pericardial attrition protocol known in the art was performed on 18 New Zealand white female rabbits weighing 3-4 kg. Bennett et al., "Next Generation Hydrogel Films as Tissue Sealants and Adhesion Barriers" - J Card Surg 18:1-6 (2003); and Wiseman et al., "Fibrinolytic Drugs to Prevent Adhesions of the Rabbit Pericardium" - J Surg Res 53:362-368 (1992).
[0310] Rabbits were sedated, placed supine, intubated and maintained under inhalation anesthesia. A median sternotomy was performed to expose the heart. The pericardium was opened and standardized superficial abrasion with dry gauze on the anterior (anterior) surface of the heart produced a "central streak" (CS...
Embodiment 2
[0314] General PEA Polymer Materials and Methods
[0315] This example represents the base material used in the following examples concerning PEA efficacy, biocompatibility.
[0316] polymer
[0317] Poly(ester amides) (PEA) are manufactured by MediVas, Inc. Poly(D,L-lactide-co-glycolide) (PLGA) was purchased from Boehringer-Ingelheim. Poly(n-butyl methacrylate) (PBMA) was purchased from Polysciences.
[0318] synthesis
[0319] PEA is prepared by synthesizing monomers of two alpha amino acids, L-leucine and L-lysine, from diols (x) and diacids (y) in the presence of hexanediol and sebacic acid. See accompanying drawing 15. The carboxyl group of the side L-lysine of the polymer chain (BnO) serves as the binding site for coupling drugs or biologics to the polymer backbone. For this study, nitrous oxide-based 4-aminoTEMPO was conjugated to PEA. See accompanying drawing 16.
[0320] cell culture
[0321] Human peripheral blood mononuclear cells were isolated by density...
Embodiment 3
[0323] macrophage development
[0324] Monocyte-to-macrophage phenotype progression and contact-induced fusion to form multinucleated cells proceeded at similar rates during the three-week culture. PEA surfaces support human monocyte adhesion and differentiation, but qualitatively, as judged by morphology and differentiation / fusion rates, PEA surfaces do not exhibit an induced hyperactivation state. See accompanying drawing 17.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com