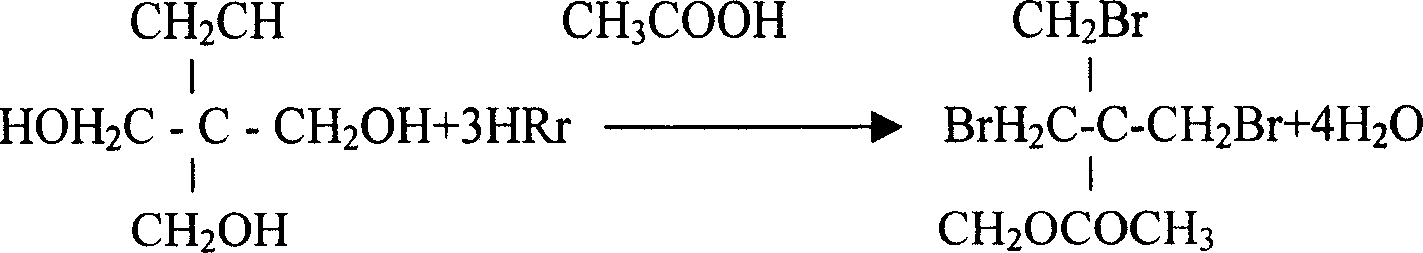Synthetic method and refining for tribromoneoamyl alcohol
A technique for the synthesis of tribromoneopentyl alcohol, which is applied in the field of synthesis of tribromoneopentyl alcohol, can solve problems such as hydrogen bromide escape, quality change, and product melting point reduction, and achieve superior performance, complete and thorough reaction, and high product quality. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
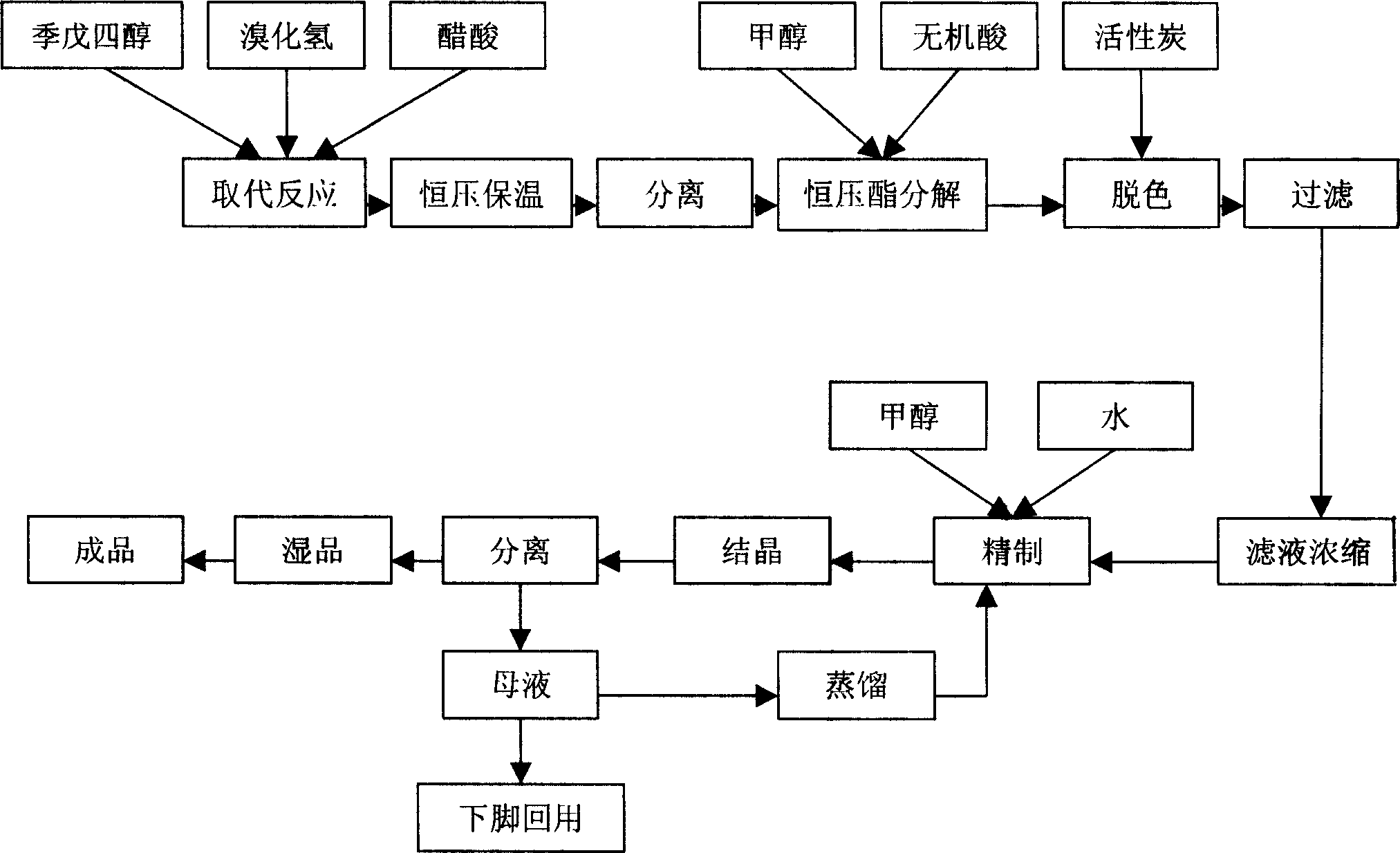
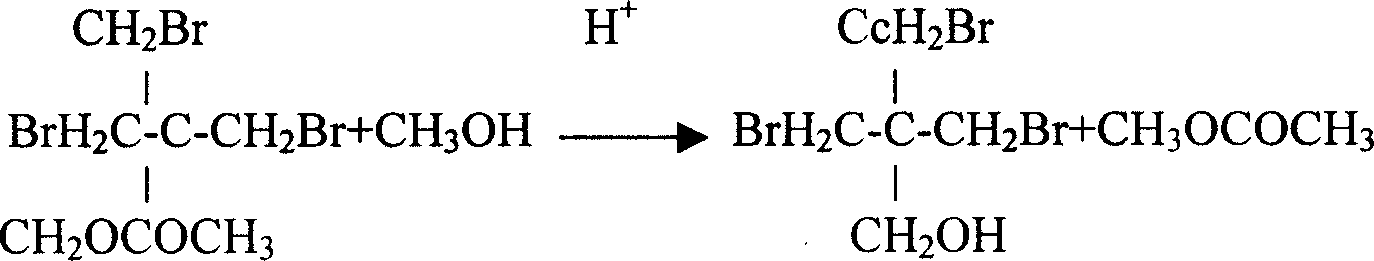
[0015] Embodiment 1, the synthetic method of tribromoneopentyl alcohol, under the acetic acid medium that concentration is more than 98%, pass into pentaerythritol and carry out substitution reaction with hydrogen bromide, the addition weight of acetic acid medium is 1.2 times of pentaerythritol weight, bromination The weight of the hydrogen introduced is twice the weight of pentaerythritol, the time for the introduction is 10 hours, and the temperature of the substitution reaction is 120°C; after the substitution reaction, it is kept at 120°C and a constant pressure of 0.6Mpa for 16 hours; after the heat preservation, it is separated Tribromoneopentyl alcohol esterification, add methanol and sulfuric acid to the tribromoneopentyl alcohol esterification and carry out constant pressure ester decomposition under the pressure of 0.5Mpa, the amount of methanol is 1.5 times of the weight of pentaerythritol, and the amount of sulfuric acid is 3% of the weight of pentaerythritol , con...
Embodiment 2
[0016] Embodiment 2, the synthetic method of tribromoneopentyl alcohol, under the acetic acid medium that concentration is more than 98%, pass into pentaerythritol and carry out substitution reaction with hydrogen bromide, the addition weight of acetic acid medium is 1.4 times of pentaerythritol weight, bromination The weight of hydrogen introduced is 2.3 times the weight of pentaerythritol, the time for passing in is 9 hours, and the temperature of the substitution reaction is 118°C; after the substitution reaction, it is kept at 118°C and a constant pressure of 0.7Mpa for 15 hours; Tribromoneopentyl alcohol esterification, adding methanol and hydrochloric acid to the tribromoneopentyl alcohol esterification and carrying out constant pressure ester decomposition under the pressure of 0.4Mpa, the amount of methanol is 1.3 times of the weight of pentaerythritol, and the amount of hydrochloric acid is 5% of the weight of pentaerythritol , constant pressure ester decomposition tem...
Embodiment 3
[0017] Embodiment 3, the synthetic method of tribromoneopentyl alcohol, under the acetic acid medium that concentration is more than 98%, pass into pentaerythritol and carry out substitution reaction with hydrogen bromide, the addition weight of acetic acid medium is 1.5 times of pentaerythritol weight, bromination The weight of hydrogen introduced is 1.8 times the weight of pentaerythritol, the time of introduction is 9.5 hours, and the temperature of the substitution reaction is 115°C; after the substitution reaction, it is kept at 125°C and a constant pressure of 0.8Mpa for 14 hours; after the heat preservation, it is separated Tribromoneopentyl alcohol esterification, adding methanol and hydrobromic acid to the tribromoneopentyl alcohol esterification under the pressure of 0.6Mpa to carry out constant pressure ester decomposition, the amount of methanol is 1.4 times the weight of pentaerythritol, and the amount of hydrobromic acid is pentaerythritol 4% by weight, the consta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com