Integrase inhibiting peptide and its application in preparing AIDS-treating medicine
A technology for inhibiting peptides and integrases, applied in peptides, antiviral agents, pharmaceutical formulations, etc., can solve problems such as peripheral nerve diseases and neutropenia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Using recombinantly expressed HIV-1 integrase as the target protein, positive phage clones were obtained by screening with phage display technology.
[0018] Phage display technology uses molecular biology techniques to clone a set of synthetic oligonucleotide fragments of a certain length into a specific expression vector, so that the expression product is presented on the surface of the phage in the form of a fusion protein. Since the peptide library contains all possible amino acid sequences of small peptides of this length, each phage surface protein presents one of the peptides, which is convenient for screening; the screened phage can be amplified by bacteria. Infect E. coli with the phage peptide library, and the random oligonucleotide fragments recombined into the phage are replicated in E. coli and expressed in the coat protein of the phage. Then, coat the target protein on the microtiter plate. After mixing the phage peptide library with the target...
Embodiment 2
[0021] Embodiment 2: Competitive inhibition ELISA experiment of synthetic heptapeptide
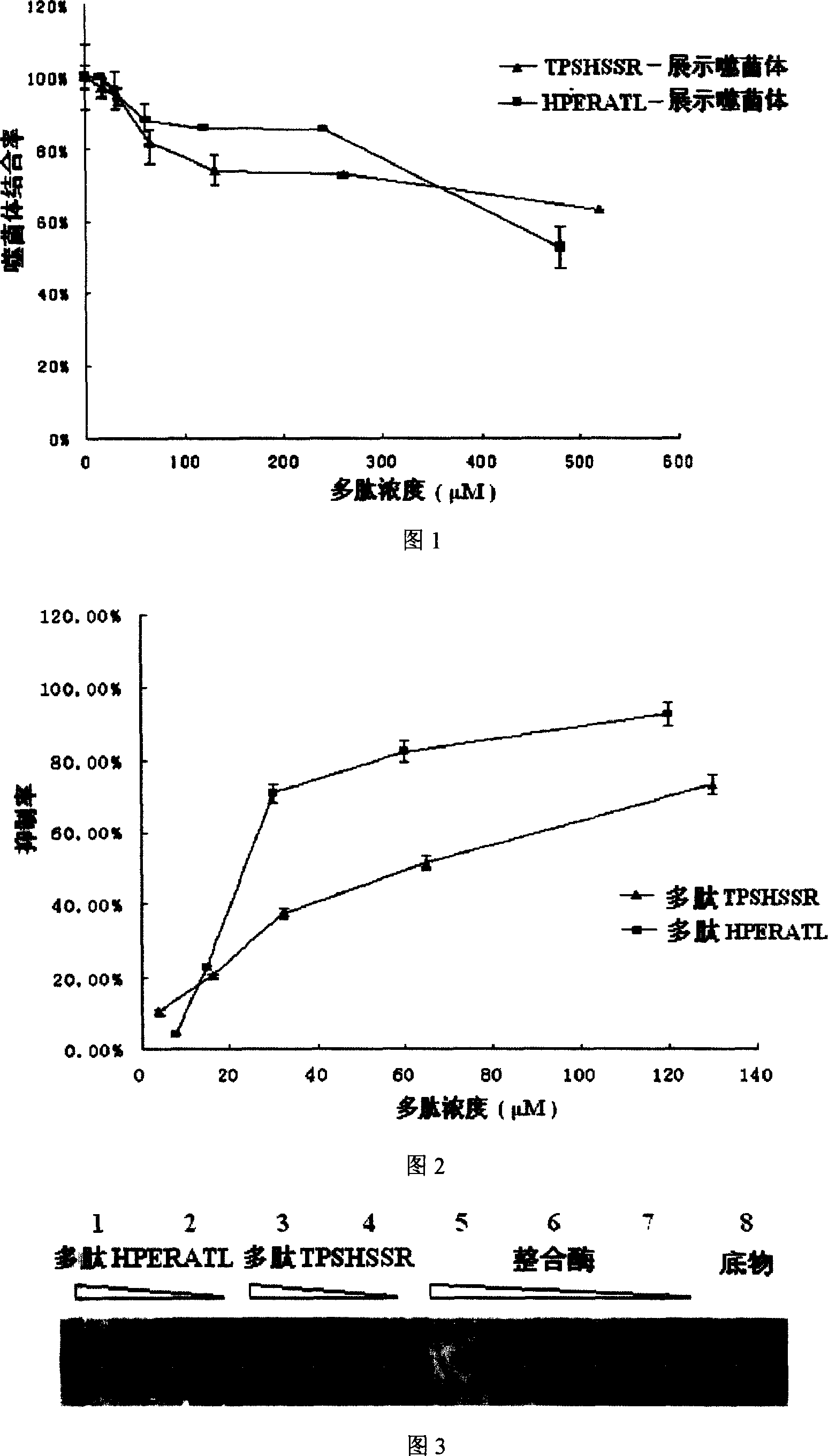
[0022] Test method: Coat 96-well plate with 100ul / well integrase, overnight at 4°C, block with 5% BSA at 37°C for 2h. TPSHSSR at different concentrations of 0, 16.25, 32.5, 65, 130, 260, and 520 μmol / L; The phages mix and compete with the coated integrase for binding. Calculate the phage binding rate: binding rate%=1-(A 450 -A' 450 ) / A 450 ×100%; where A 450 Absorbance at 450nm wavelength without inhibitor, A' 450 Absorbance at a wavelength of 450 nm after adding the inhibitor.
[0023] Test results: With the increase of the heptapeptide added, the binding rate of the phage gradually decreased, that is, the competitive inhibition rate of the corresponding polypeptide increased. See Figure 1. With the increase of the concentration of the synthesized heptapeptide, the corresponding displayed phage and integrase binding rate decreased. The results indicated that these two peptides had...
Embodiment 3
[0024] Embodiment 3: Synthetic heptapeptide inhibition integrase integration activity experiment
[0025] Test method: First, the plasmid pBluescript SK (+) was digested with Sma I at 30°C overnight, and the product was subjected to 1% agarose gel electrophoresis, and the digested product was recovered by cutting the gel, and dissolved in the coating solution (20mmol / L Tris-HCl pH7.4, 20mmol / L EDTA, 2mol / L NaCl), the final concentration of DNA is 2ug / ml; use it to coat a 96-well plate, 100μl per well for 2h at 37°C, or overnight at 4°C. The donor DNA was annealed to two single-stranded oligonucleotide chains (UV5BR: 5'-Biotin-GTGTGGAAAATCTCTAGCAGT-3', UV5: 5'-ACTGCTAGAGATTTTCCACAC-3') synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. become. VU5BR / VU5 was mixed in TEN buffer (10mmol / L Tris-HCl pH8.0, 1mmol / L EDTA pH8.0, 0.1mol / L NaCl) at a ratio of 1:1.2, heated at 80°C for 3min, and slowly cooled to room temperature. Store in a 4°C refrigerator. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com