Method for detecting optical self-cleaning material photocatalysis performance by fluorophotometry
A technology for photocatalysis and detection of light, which is applied in the directions of fluorescence/phosphorescence, material excitation analysis, preparation of test samples, etc. It can solve the problems of long time consumption and low sensitivity, and achieve the effect of high sensitivity and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
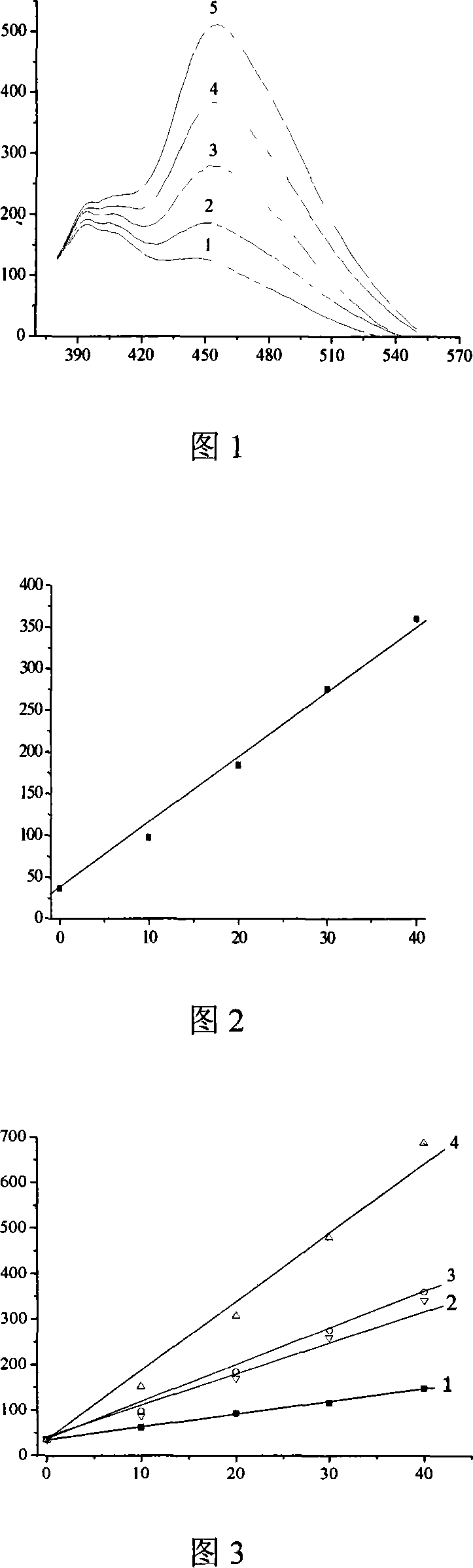
[0024] Example 1: Cut 4 kinds of commercial light self-cleaning glass: A, B, C, D into 4×3cm 2 of small pieces. Take 2 pieces of each light self-cleaning glass (24cm 2 ) were placed flat in a petri dish (simple reactor) with a diameter of 9cm, and the reactor was placed directly below a 9W ultraviolet lamp (λmax=254nm) (distance 20cm), and 20mL of coumarin (1.0×10 -3 mol / L, pH=3), turn on the light source, and photocatalytically oxidize coumarin. Take out 2mL of the solution every 10min and use a fluorescence spectrophotometer to detect the fluorescence intensity of the solution at an excitation wavelength of 346nm and a fluorescence wavelength of 456nm (slit width: Ex, Em: 5nm; sensitivity: high). Repeat 4 times. The fluorescence intensities measured under the above-mentioned conditions of these four kinds of optical self-gu glasses are plotted against their illumination time respectively, as shown in Figure 2, which are all straight lines; but the slopes K of these straig...
example 2
[0025] Example 2: Cut 4 kinds of commercial light self-cleaning glass: A, B, C, D into 4×3cm 2 of small pieces. Take 2 pieces of each light self-cleaning glass (24cm 2 ) were placed flat on a petri dish (simple reactor) with a diameter of 9cm, and the reactor was placed directly below a 9W ultraviolet lamp (λmax=254nm) (distance 15cm), and 30mL rhodamine B (5.0×10 -4mol / L, pH=6), turn on the light source, and photocatalytically degrade Rhodamine B. Take out 2mL of the solution every 10min and use a fluorescence spectrophotometer to detect the fluorescence intensity of the solution at an excitation wavelength of 550nm and a fluorescence wavelength of 573nm (slit width: Ex, Em: 5nm; sensitivity: high). Repeat 4 times. The fluorescence intensities of these four kinds of self-cleaning glasses measured under the above conditions are plotted against the illumination time, and they are all straight lines; however, the slopes K of these straight lines are different. The order of p...
example 3
[0026] Example 3: Cut 3 kinds of commercial light self-cleaning ceramics: E, F, G into 3×3cm 2 of small pieces. Take 4 pieces of each (36cm 2 ) were placed flat in a petri dish (simple reactor) with a diameter of 9cm, and the reactor was placed directly under a 9W ultraviolet lamp (λmax=254nm) (distance 15cm), and 50mL of 8-hydroxyquinoline aluminum (2.0× 10 -4 mol / L, pH=4), turn on the light source, and photocatalytically degrade 8-hydroxyquinoline aluminum. Take out 2mL of solution every 10min and use a fluorescence spectrophotometer to detect the fluorescence intensity of the solution at an excitation wavelength of 360nm and a fluorescence wavelength of 500nm (slit width: Ex, Em: 10nm; sensitivity: mid). Repeat 4 times. The fluorescence intensities measured under the above conditions for the three kinds of self-cleaning ceramics were plotted against the illumination time, and they were all straight lines. The order of catalytic activity is: E>F>G.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com