Method of reducing serum proinsulin levels in type 2 diabetics
A technology for type 2 diabetes and serum insulin, applied in metabolic diseases, pharmaceutical formulations, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1. Pulmonary Delivery of Technosphere to Rats / Insulin produces rapid absorption
[0023] Pulmonary Technosphere administered as a dry powder aerosol in rats / The pharmacokinetic (PK) profile of insulin particles compared to the PK profile of human insulin delivered by subcutaneous (s.c.) injection. The aerosol was administered using a flow-past, nose-only inhalation contact system. In the first experiment, all animals received the same formulation (9.1% insulin) but the duration of dosing was adjusted so that doses of approximately 1 IU and 3 IU were delivered per rat (200 g body weight). A linear dose-dependent response was observed: maximum serum insulin concentration (C MAX ) for a dose of 0.9IU of Technosphere 76±12 μIU / ml after insulin and 240±49 μIU / ml after the 2.7 IU dose. Maximum serum insulin levels were obtained in samples taken immediately after dosing was complete, shows Technosphere / Insulin is rapidly absorbed into the systemic cir...
Embodiment 2
[0026] Example 2. In the primary cell culture model of alveolar epithelium, Technosphere Fumaryl diketopiperazine particles enhance insulin absorption without signs of cytotoxicity
[0027] To study the Technosphere / Mechanisms of insulin production through the epithelial barrier deep in the lung, experiments were performed using primary cultured monolayers of rat alveolar epithelial cells. Alveolar type II cells were isolated and cultured on semipermeable polycarbonate membranes until forming a compact monolayer with high transepithelial electrical resistance (TEER). Then use Technosphere at 37°C Insulin transport experiments across these cell monolayers were performed in an apical-to-basal orientation for insulin product and unformulated insulin controls. Insulin exhibited 1.90 ± 0.34 × 10 -8 Apparent permeability in cm / s (P app ), while Technosphere / insulin product exhibited 2.08±0.82×10 -7 Ten times higher P of cm / s app . There was no measurable change in...
Embodiment 3
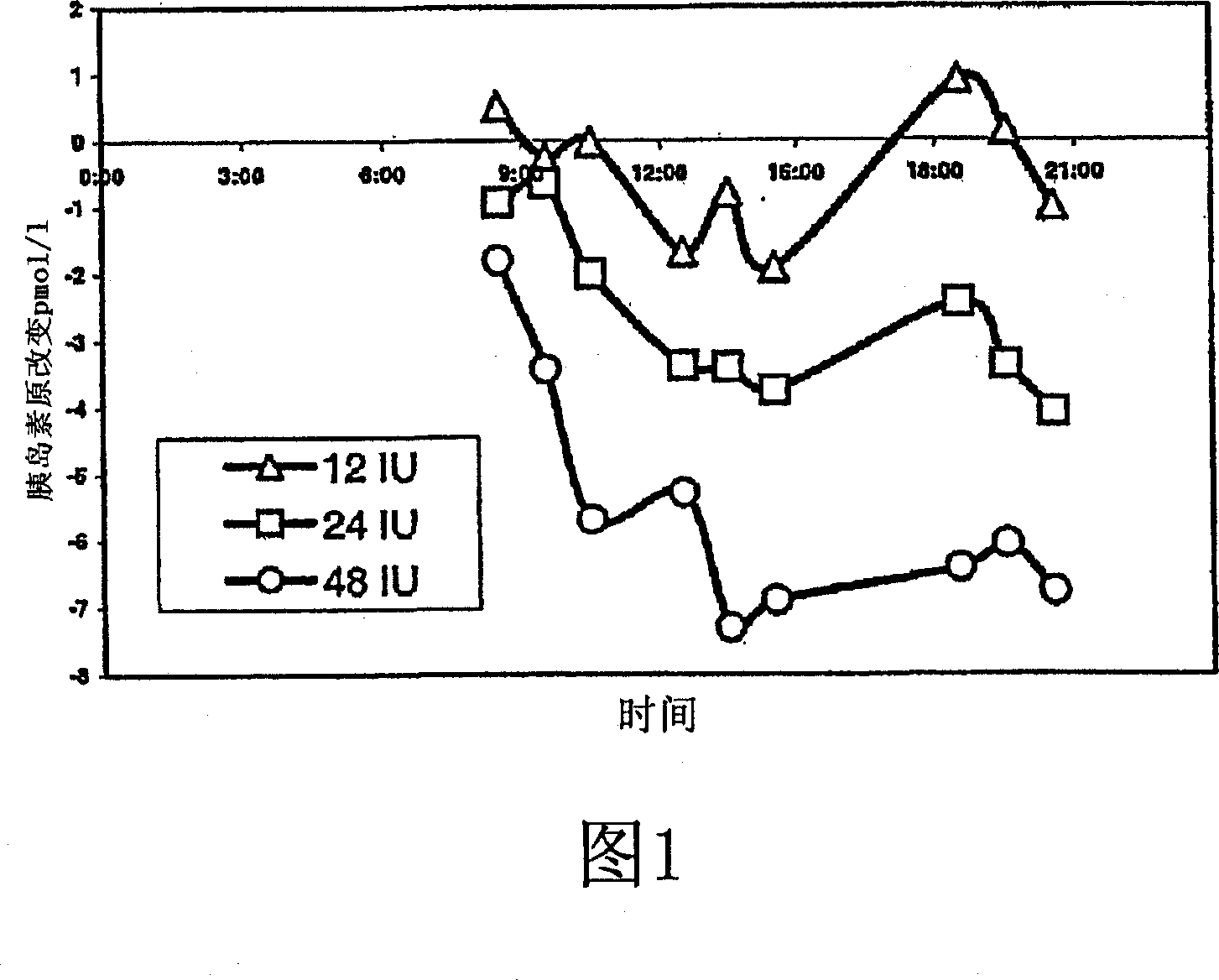
[0028] Example 3. Treatment of Humans with Transpulmonary Insulin to Reduce Serum Proinsulin Levels Technosphere Inhalation of insulin / insulin (TI) provided a rise in serum insulin comparable to the first phase response. This study investigated the pharmacodynamics of TI and its effect on natural proinsulin release (iPi release). Twenty-four patients with type 2 diabetes received Technosphere with 4 different loadings of insulin (i.e., 0, 12 IU, 24 IU, or 48 IU of recombinant regular human insulin) five minutes after the start of a standardized meal on alternate study days. Dosage of substrate. Blood glucose (BG), serum insulin and serum iPi were measured before (0 min), 60 and 120 min after the start of each meal.
[0029] TI reduces dietary BG levels in a dose-dependent manner. BG (mg / dl) (±SD) sixty minutes after lunch was: placebo, 183.2 (±44.4); 12IU, 170.8 (±30.5) (p=0.266); 24IU, 156.3 (±31.9) (p= 0.020); and 48 IU, 132.6 (29.1) (p<0.001). All doses caused an i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com