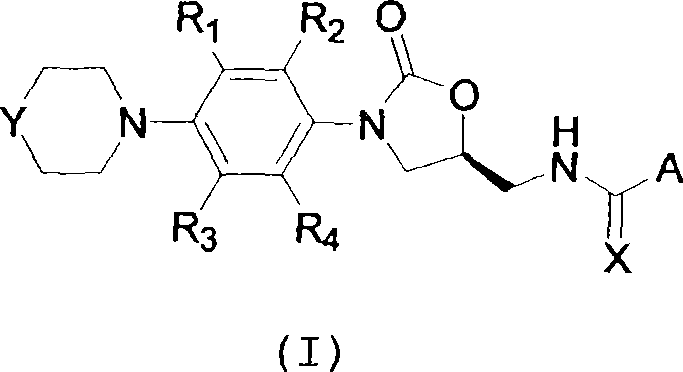
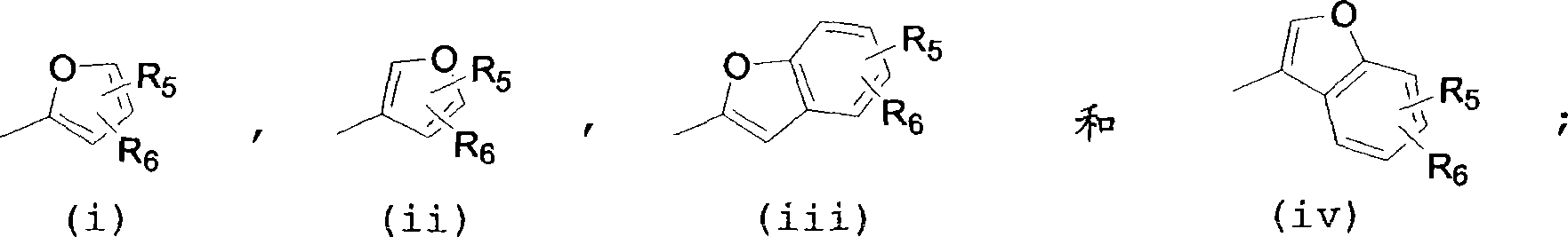
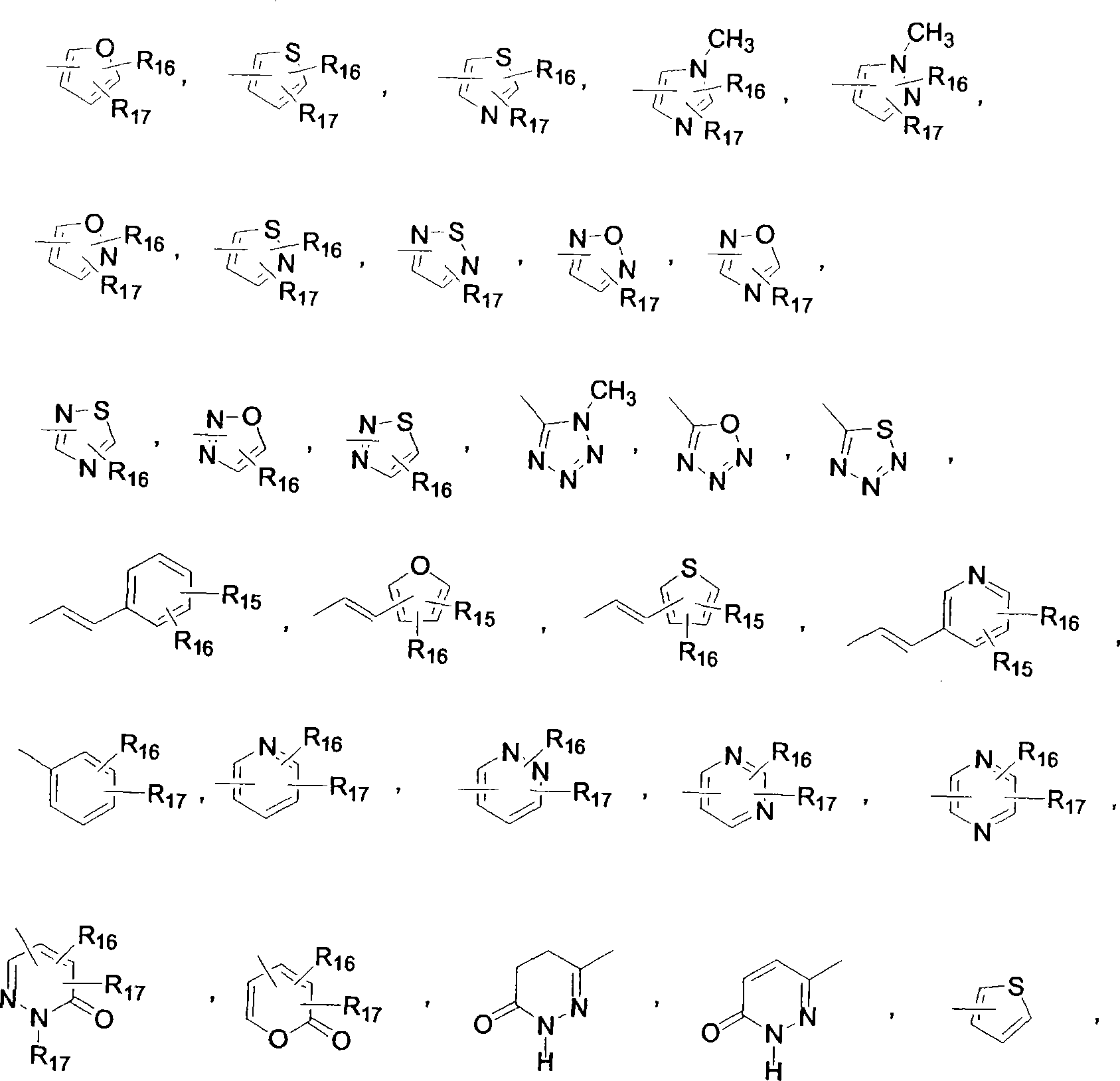Oxazolidinone compounds and compositions and methods related thereto
A compound and alkyl technology, applied in the field of oxazolidinone antibacterial compounds, can solve the problems of undescribed antibacterial activity and MAOi activity, and achieve the effect of weak inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] (a) Preparation of amide compound (I, X=O):
[0108] Formally, the amides are prepared by condensation of the active form of the acid (III) with the corresponding aminomethyl derivative (II). The acid can previously be converted to a reactive acylating reagent either by isolation or by in situ preparation. Acid halides, imidazolides, p-nitrophenyl esters or 2,4,5-trichlorophenyl esters are more commonly used isolatable acylated species prepared directly from carboxylic acids. These activation methods generate acid halides in situ in the presence of nucleophiles such as refluxing carboxylic acids, triphenylphosphine, trichloromethyl bromide, and the amines. Other coupling reagents convert carboxylic acids into reactive intermediates that react with nucleophilic amines. Many such reagents can be used, some of them are as follows: dicyclohexylcarbodiimide, 2-chloropyridinium cation, 3-chloroisoxazolium cation, diphenylphosphoryl Azide, N-hydroxybenzotriazole (HOBt), 2-(...
Embodiment 1
[0145] Example 1: N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)-phenyl]-2-oxo-5-oxazolidinyl]methyl]furan-2 -yl-amide
[0146]
[0147] In 5 ml of dichloromethane (DCM), 57 mg (1.5 equivalents) of 2-furanoic acid, 21 mg (0.5 equivalents) of 4-(dimethylamino)pyridine (DMAP), 97 mg of 1-ethyl-3-(3′-dimethyl A solution of aminopropyl)carbodiimide hydrochloride (EDCI.HCl, 1.5 equiv) was stirred at room temperature under argon for 30 minutes. Then, 100 mg (1 equivalent) of N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)-phenyl]-2-oxo-5-oxazole was added to 5 ml DCM Alkyl]methyl]amine and stirring was continued for 12 hours when complete conversion of the starting amine was observed by TLC. The crude mixture was washed with 5% HOAc solution, saturated NaHCO 3 and washed with brine. The combined organic layers were dried (MgSO 4 ) and concentrated under vacuum to give 125 mg of N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)-phenyl]-2-oxo-5-oxazolidinyl] Methyl]furan-2-yl-amide (Yield = 95%).
[01...
Embodiment 2
[0151] Example 2: N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)-phenyl]-2-oxo-5-oxazolidinyl]methyl]furan-2 -yl-thioamide
[0152]
[0153] 87mg N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)-phenyl]-2-oxo-5-oxazole in 4ml 1,4-dioxane A solution of alkyl]methyl]furan-2-yl-amide, 271.3 mg (3 eq) of Lawesson's reagent was heated at 65°C for 3 hours and at 100°C for 1 h. The solvent was removed under reduced pressure and the crude product was purified by column chromatography (Merck silica gel, DCM / MeOH 99 / 1) to obtain 87 mg of the title product (Yield = 96%).
[0154] HPLC (t, %): 11.3 min, 96%.
[0155] MS(ESI)m / z=406(M+1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com