FGFR binding peptides
A technology for peptide sequences and compounds, applied in the field of new peptide compounds, can solve the problems of direct binding and activation of FGFRs that do not show sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0271] The preparation of polyclonal antibodies is well known to those skilled in the art. See, for example, Green et al. 1992. Production of Polyclonal Antisera in Immunochemical Protocols (Manson ed.), pp. 1-5 (Humana Press); Coligan et al., Production of Polyclonal Antisera in Rabbits, Rats Mice and Hamsters, in Current Protocols inImmunology, Section 2.4.1, which is hereby incorporated by reference.
[0272] Likewise, the preparation of monoclonal antibodies is routine. See, eg, Kohler and Milstein, Nature, 256:495-7 (1975); Coligan et al., Sections 2.5.1-2.6.7; and Harlow et al., in Antibodies: A Laboratory Manual, page 726, Cold Spring Harbor Pub. (1988). Monoclonal antibodies can be isolated and purified from hybridoma cultures by a variety of established techniques. Such separation techniques include Protein-A Sepharose affinity chromatography, size exclusion chromatography, and ion exchange chromatography, see, e.g., Coligan et al., Sections 2.7.1-2.7.12 and Sectio...
Embodiment 1
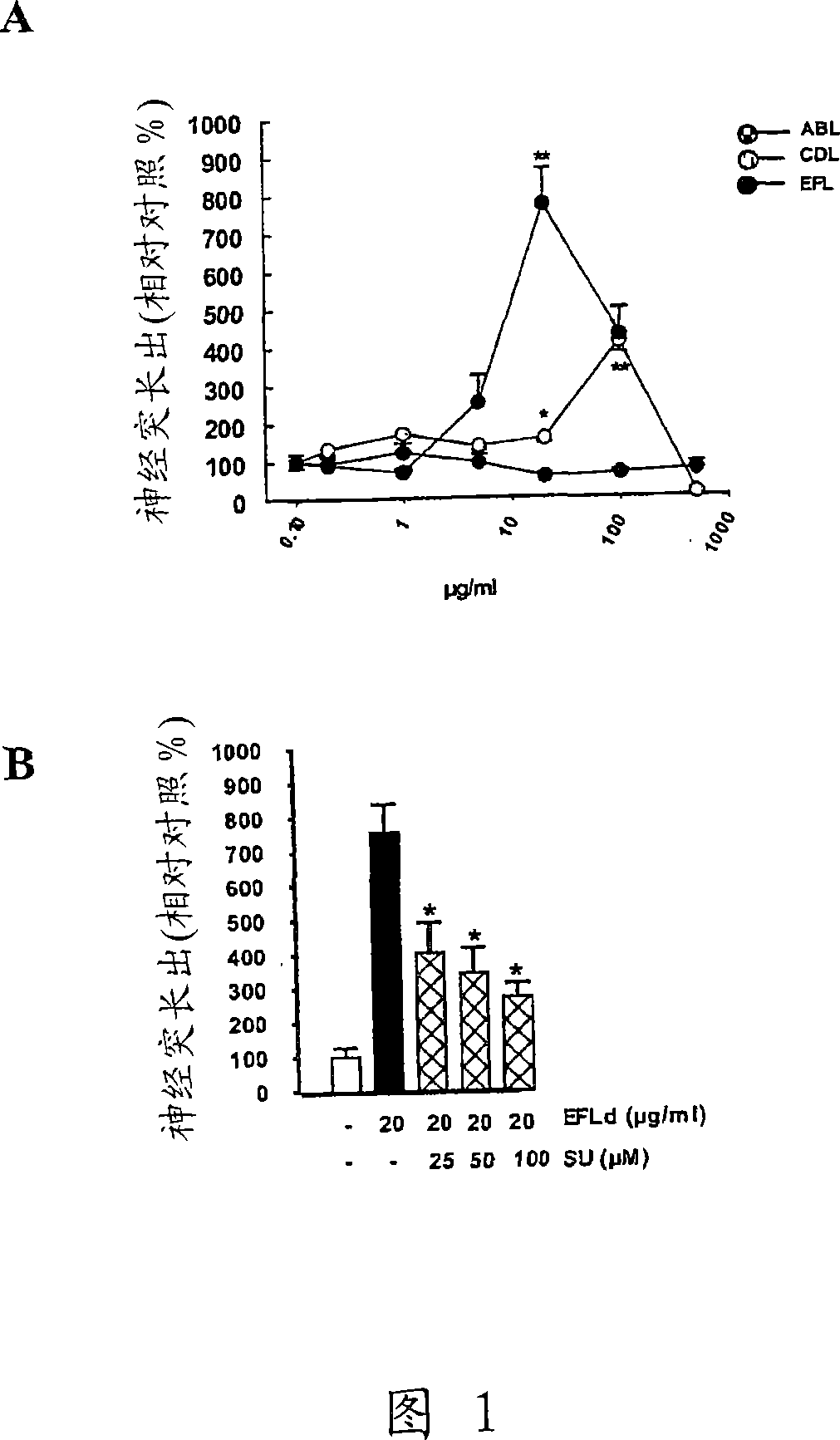
[0417] Example 1 Stimulation of neurite outgrowth
[0418] Cerebellar granule neurons (CGN) were prepared from postnatal 7 day Wistar rats essentially as described previously (Neiiendam et al., (2004) J Neurochem. 91(4):920-35). Cerebellar tissue was dissected in modified Krebs-Ringer solution kept on ice and processed as described to obtain upper hippocampal neurons. All cell cultures were maintained at 37 °C with 5% CO 2 Incubate under a humidified atmosphere. All animals were handled in accordance with national guidelines for animal welfare.
[0419] Dissociated cells at 10,000 cells / cm 2Spread on uncoated 8-well permanox Lab-Tek culture slides (chamber slides), add 0.4% (w / v) bovine serum albumin (BSA; Sigma-Aldrich), 2% (v / v) In Neurobasal medium with B27 Neurobasal supplement, 1% (v / v) glutamax, 100 U / ml penicillin, 100 μg / ml streptomycin and 2% 1M HEPES (all from Gibco, BRL). Add peptide solutions with or without multiple signal transduction pathway inhibitors to 3...
Embodiment 2
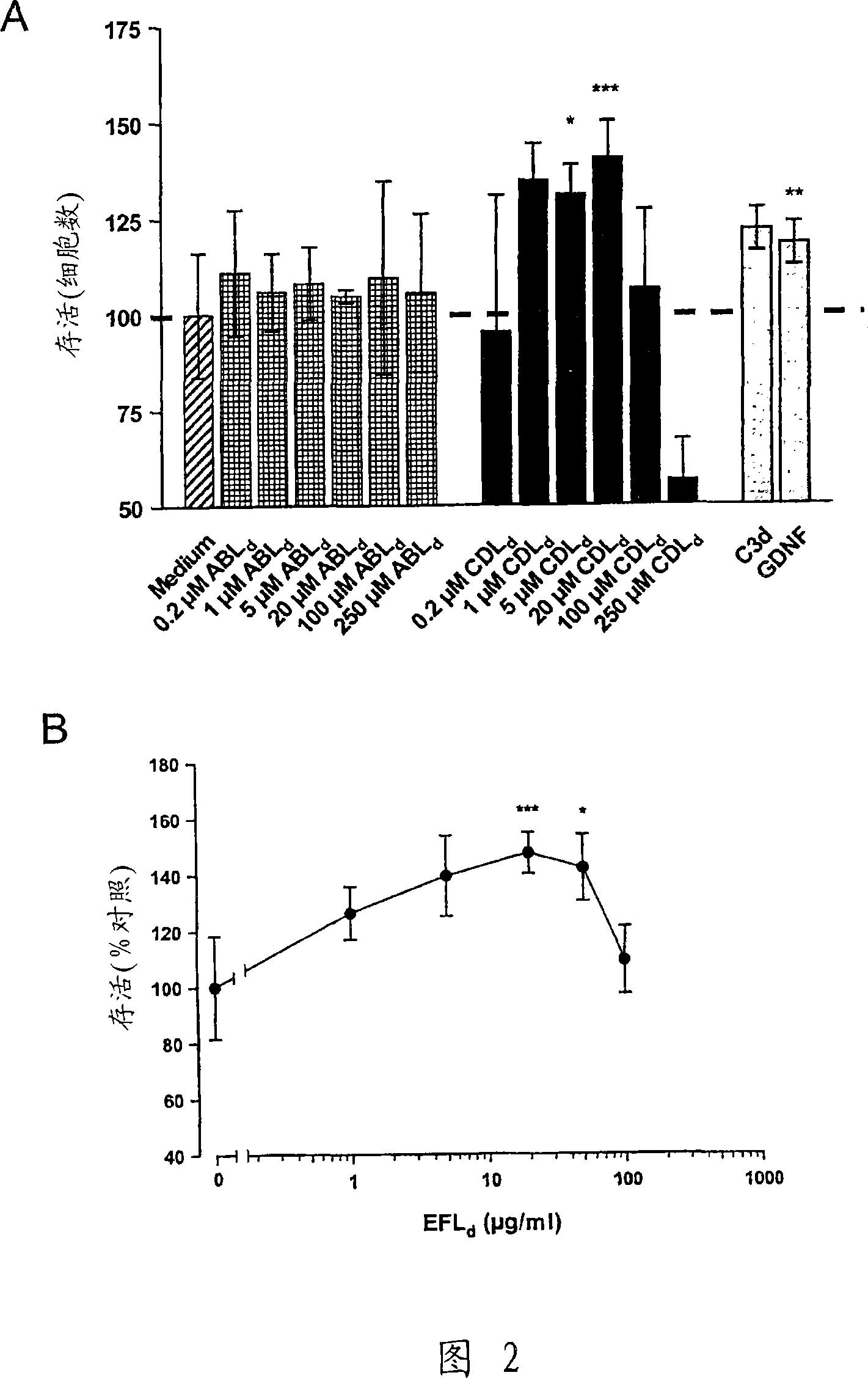
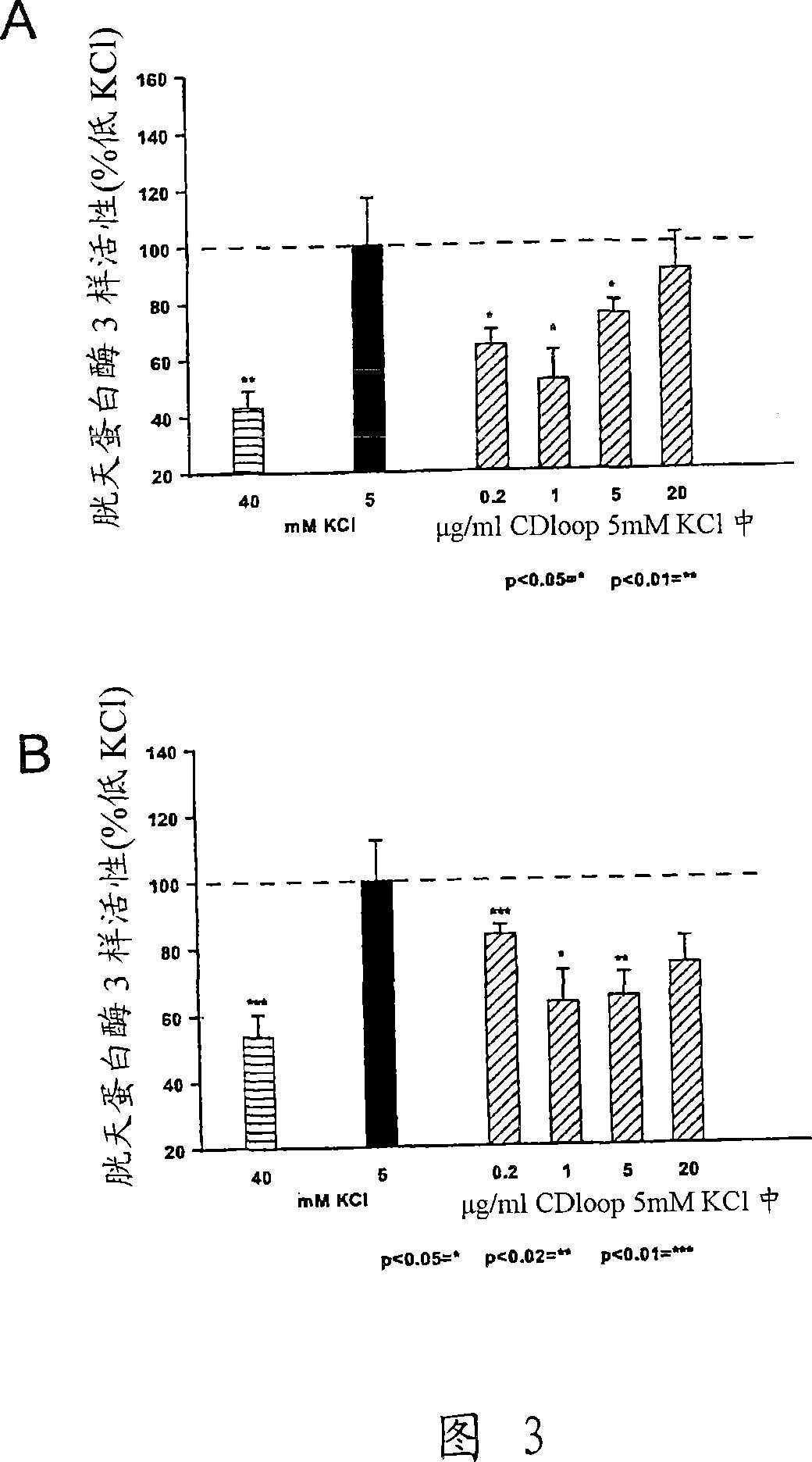
[0421] Example 2 Stimulation of Neuron Survival
[0422] Survival assay
[0423] The primary cultured CGN was cultured at 100,000 cells / cm 2 The density was spread on polylysine-coated 8-well permanox slides, added with 2% (v / v) B27, 0.5% (v / v) glutamax, 100U / ml penicillin, 100μg / ml streptomycin and In Neurobasal-A medium (Gibco, BRL) with KCl, the final concentration of KCl in the medium was 40 mM. 24 hours after plating, cytosine-β-D-arabinofuranoside (Ara-C; Sigma-Aldrich) was added to a final concentration of 10 μM to avoid glial cell growth, and neurons were allowed to redifferentiate at 37°C for 6 days. Wash 2 times and change medium to supplemented with 1% (v / v) glutamine, 100 U / ml penicillin and 100 μg / ml streptomycin, 3.5 g D-glucose / l and 1% (v / v) acetone Sodium bicarbonate (Gibco BRL) and Eagle's minimal medium (BME; Gibco BRL) with different concentrations of peptides were used to induce apoptotic cell death. The potassium concentration in the culture was thus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com