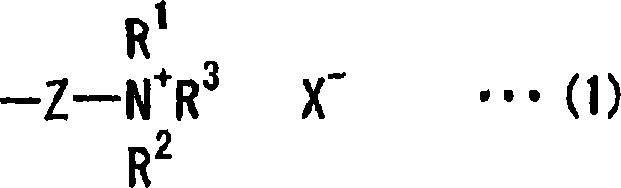Lithographic printing plate precursor
一种印版前体、平版的技术,应用在负片型或正片型平版印版前体领域,能够解决感光层亲合力不总是优异、非图像区域污染、损害等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] step 1:
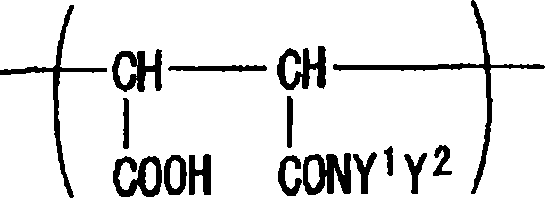
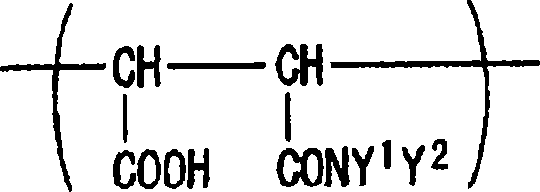
[0179] In a 200 ml reaction vessel equipped with a reflux condenser, a thermometer, a dropping device and a stirrer, 25.0 g of maleic anhydride copolymer GS-601 (Gifu Shellac Mfg., Co., Ltd.: monomer ratio, Maleic anhydride / styrene=40 / 60, Mwt=5,500) and 75.0 g of dimethylacetamide. After heating to 80°C, a solution mixture of 10.0 g of N,N-dimethylaminopropylamine and 15.0 g of dimethylacetamide was added to the reaction vessel. After completion of the dropwise addition, the internal temperature was kept at 80° C. for 30 minutes to obtain a maleamic acid copolymer. After the reaction was complete, a small sample was collected and checked for solubility in ethyl acetate or water. As a result, the maleamic acid copolymer was insoluble in neither ethyl acetate nor water.
[0180] Step 2:
[0181] After completing the reaction of Step 1, the reaction mixture was heated and a solution mixture of 12.0 g of bromopropane and 15.0 g of dimethylacetamide was added d...
Embodiment 2-19
[0185] In the same manner as in Synthesis Example 1, N-substituted maleamic acid copolymers in which hydrogen atoms on nitrogen atoms were substituted with groups were obtained from various maleic anhydride copolymers. The results are shown in Table 1.
[0186] Table 1: Synthesis examples of maleamic acid copolymers in which hydrogen atoms on nitrogen atoms are replaced by groups
[0187] Maleic anhydride copolymer
N-substituted maleamic acid copolymer
Maan
Stylized
αMSty
AN
Mwt
Yield (%)
Synthesis Example 2
RV-89
25
75
6000
86
WS
Synthesis Example 3
RV-11
20
80
8000
70
WS / WD
Synthesis Example 4
RV-12
20
40
40
6000
68
WD
Synthesis Example 5
RV-13
20
50
30
8000
72
WS
Synthesis Example 6
RV-14
20
...
Embodiment 20
[0198] In a 200ml flask equipped with reflux condenser, thermometer, dropping device, stirrer and gas inlet, introduce 3.75g maleic anhydride monomer, 13.75g styrene monomer, 7.5g acrylonitrile monomer and 75.0g dimethyl acetamide. After bubbling nitrogen for one hour and heating to 80° C., 0.2 g of 2,2′-azobisisobutyronitrile (AIBN) were added. The reaction was carried out for 3 hours while adding 0.2 g of AIBN every 30 minutes. After completing the reaction, the reaction mixture was cooled and the viscosity of the reaction mixture was measured. As a result, it was VIS A (Gardener viscosity indicator: 25°C). The reaction mixture was soluble in ethyl acetate but insoluble in water.
[0199] While maintaining at 80°C without taking the reaction mixture out of the vessel, a solution mixture of 3.75 g of N,N-dimethylaminopropylamine and 15.0 g of dimethylacetamide was added dropwise. After the dropwise addition was completed, the reaction was continued at 80° C. for 30 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com