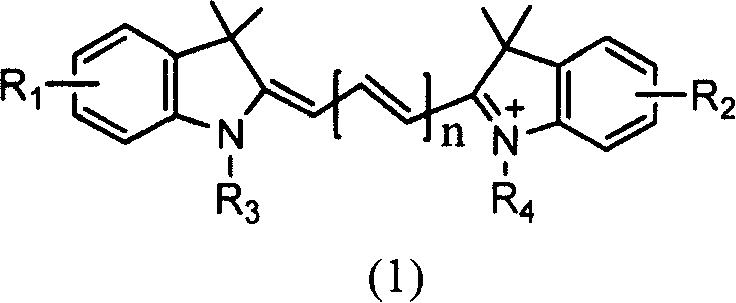
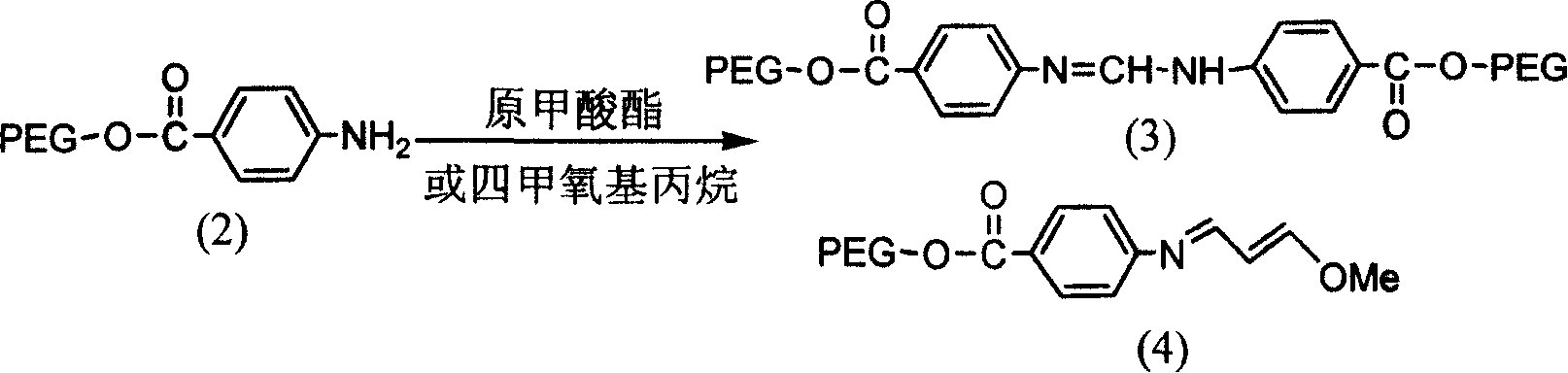
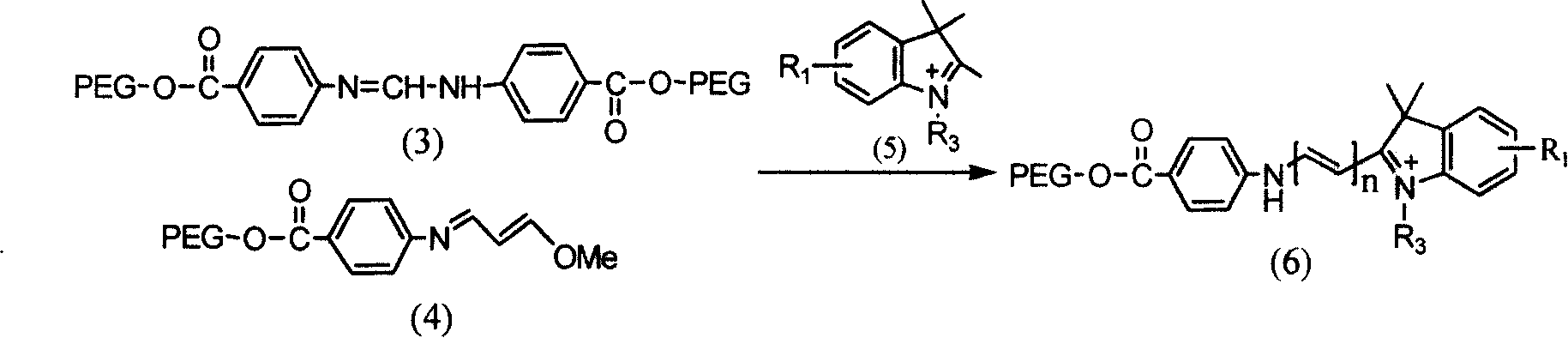
Solid-phase synthesis of asymmetric indocyanine dyes
A solid-phase synthesis, indocyanine technology, applied in the direction of organic dyes, chemical instruments and methods, methine/polymethine dyes, etc., can solve the problems of difficult purification, complicated operation, low yield, etc., to avoid by-products , The effect of increasing the purity of the product and simplifying the purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0031] The synthetic steps of unsymmetrical indocyanine dye Cy3 are as follows:
[0032] Step 1. Preparation of Polyethylene Glycol-2000 Supported Monomethyl Electrophile
[0033] 3.02 g (1.35 mmol) of aniline loaded on polyethylene glycol-2000 and 10 ml (60 mmol) of triethyl orthoformate were dissolved in 27 ml of glacial acetic acid, and reacted at 55° C. for 5.5 hours under electromagnetic stirring. After cooling to room temperature, 50ml of ether was added to the reaction liquid, stirred electromagnetically for 5 minutes, then allowed to stand for 10-20 minutes, and filtered to obtain 2.43g of primary methyl electrophile loaded on polyethylene glycol-2000 as a pale yellow solid. The rate is 80.1%. IR (KBr): v3446, 2884, 1701, 1638, 1604, 1529cm-1 . 1 H NMR (300MHz, CDCl 3 ): δ8.53 (s, 1H), 8.14 (d, 4H), 7.27 (d, 4H), 3.3-4.5 (m, polyethylene glycol). The reaction formula is as follows:
[0034]
[0035] PEG in the above reaction formula is polyethylene glycol carri...
preparation Embodiment 2
[0043] The synthetic steps of unsymmetrical indocyanine dye Cy5 are as follows:
[0044] Step 1 Preparation of polyethylene glycol-2000-loaded trimethyl electrophile
[0045] Dissolve 2.02g (0.90mmol) of aniline loaded on polyethylene glycol-2000, 3.7mL (22.2mmol) of 1,1,3,3-tetramethoxypropane in 9mL of glacial acetic acid, and react at 55°C for 5.5 hours under electromagnetic stirring . After cooling to room temperature, 30 mL of diethyl ether was added to the reaction solution, stirred electromagnetically for 5 minutes, then allowed to stand for 10 to 20 minutes, and filtered to obtain 1.56 g of trimethyl electrophile loaded on polyethylene glycol-2000 as a yellow solid, with a yield of 73.0%. . IR (KBr): v3437, 2886, 1706, 1639, 1601, 1465cm -1 . 1 H NMR (300MHz, CDCl 3 ): δ8.06 (m, 3H), 7.85 (d, 1H), 7.11 (d, 2H), 6.65 (dd, 1H), 3.3-4.5 (m, polyethylene glycol). The reaction equation is as follows:
[0046]
[0047] Step 2 Preparation of Polyethylene Glycol-2000...
preparation Embodiment 3
[0054] The synthetic steps of unsymmetrical indocyanine dye Cy3 are as follows:
[0055] Step 1 Preparation of Polyethylene Glycol-1000-loaded Monomethyl Electrophile
[0056] 1.67 g (1.35 mmol) of aniline loaded on polyethylene glycol-1000 and 10 mL (60 mmol) of triethyl orthoformate were dissolved in 27 mL of glacial acetic acid, and reacted at 55° C. for 5.5 hours under electromagnetic stirring. Cool in an ice bath to 0-5°C, then add 50 mL of diethyl ether to the reaction solution, stir it electromagnetically for 5 minutes, then continue to stand in the ice bath for 10-20 minutes, and filter to obtain a light yellow solid polyethylene glycol-1000-loaded primary Methyl electrophile 1.20g, yield 71.2%.
[0057] Step 2 Preparation of Polyethylene Glycol-1000 Loaded Dimethyl Hemicyanine Dye
[0058] 1.31g (1.05mmol) of primary methyl electrophile loaded on polyethylene glycol-1000, 0.28g (1.05mmol) of 1-ethyl-2,3,3-trimethylindole-5-sulfonate and 0.34 mL (2.08 mmol) of triet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com