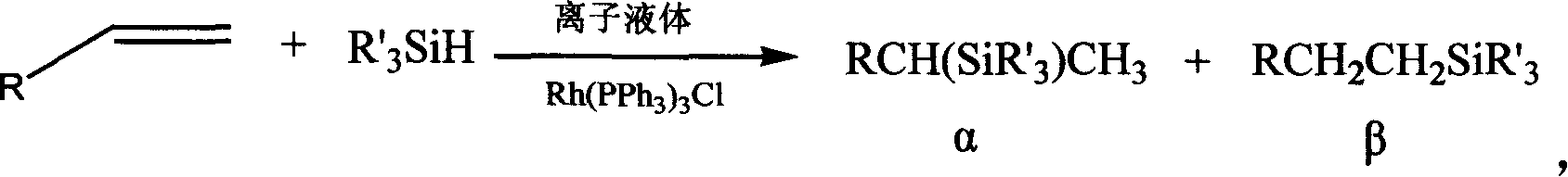Silicon-hydrogen additive reaction method
A technology of hydrosilylation reaction and hydrosilane, which is applied in the direction of chemical instruments and methods, silicon organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., which can solve the problems of difficult separation, recovery and reuse, and catalytic reactivity It is not high, it is difficult to apply to problems such as industrialization, to achieve high thermal and chemical stability, simple operation of reaction and product separation, and the effect of improving activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
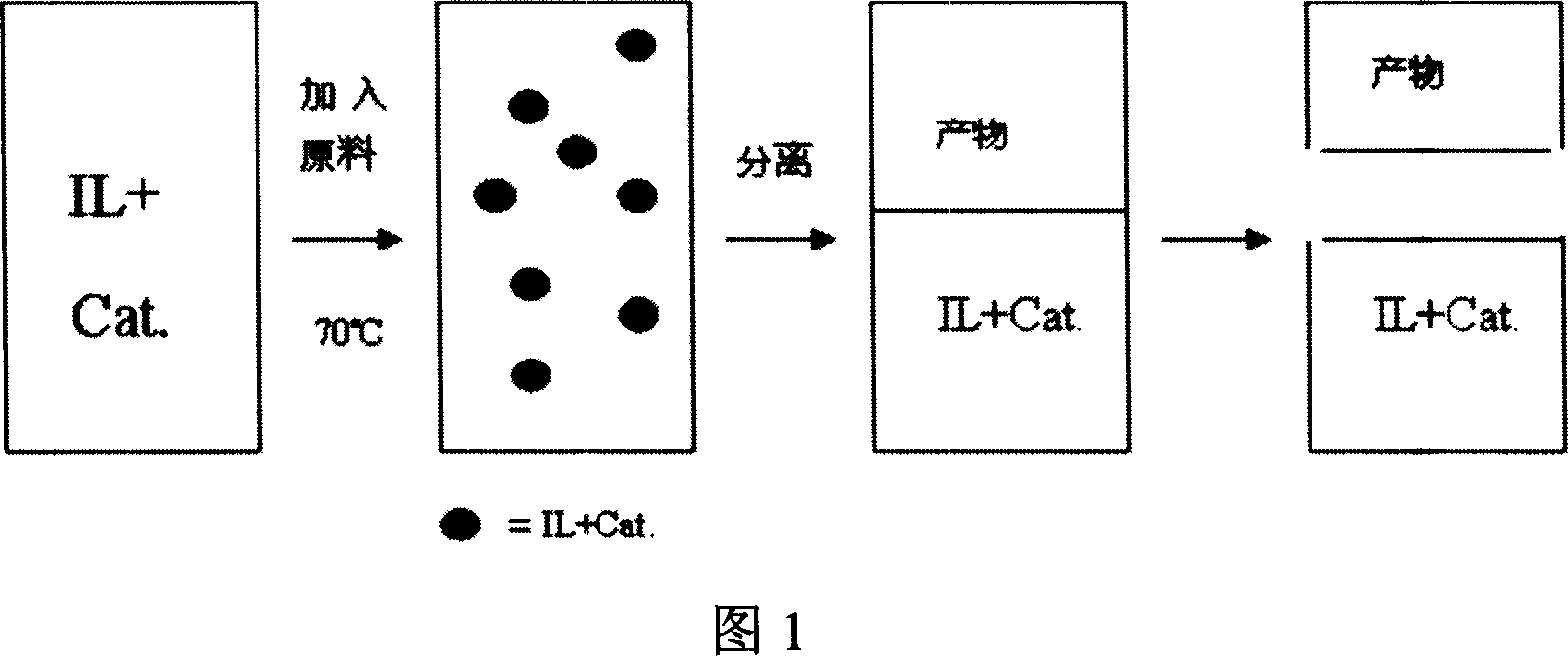
Image
Examples
Embodiment 1
[0023] In a 250 ml three-necked flask, triphenylphosphine rhodium chloride (1.25 mmol) and 1-butyl-3-methylimidazolium hexafluorophosphate (BMImPF 6 ) (25mL), under the protection of nitrogen, the temperature was slowly raised to 70°C, the reaction was stirred for 0.5 hours, the condensing reflux was opened, and hexene (1.25mol) and triethoxyhydrogensilane (1.25mol) were successively added dropwise through the dropping funnel, keeping Reaction temperature, continue to stir and react for 5 hours, let stand, cool to room temperature, decant to separate the upper layer product, measure the conversion rate of hexene by GC-MS 100%, β adduct 1-triethoxysilyl Hexane (CH 3 (CH 2 ) 5 Si(OCH 2 CH 3 ) 3 ) The yield is 97.3%.
Embodiment 2
[0025] In Example 1, triphenylphosphine rhodium chloride (6.25mmol) was added, and the conversion rate of hexene measured by GC-MS after the reaction was 100%, and the yield of β-adduct 1-triethoxysilyl hexane The rate is 99.6%.
Embodiment 3
[0027] In Example 1, triphenylphosphine rhodium chloride (3.12mmol) was added, and the conversion rate of hexene measured by GC-MS after the reaction was 100%, and the yield of β-adduct 1-triethoxysilyl hexane The rate is 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com