Benzo-phenanthroline derivative and application thereof
A technology of benzophenanthroline and derivatives, which is applied in the field of organic electroluminescence devices, can solve the problem that the molecular energy gap and triplet energy level cannot be effectively improved, the host bipolar characteristics cannot be realized, and the lack of electron donating groups can be solved. and other problems, to achieve the effects of high carrier transport and luminous efficiency, high melting point and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
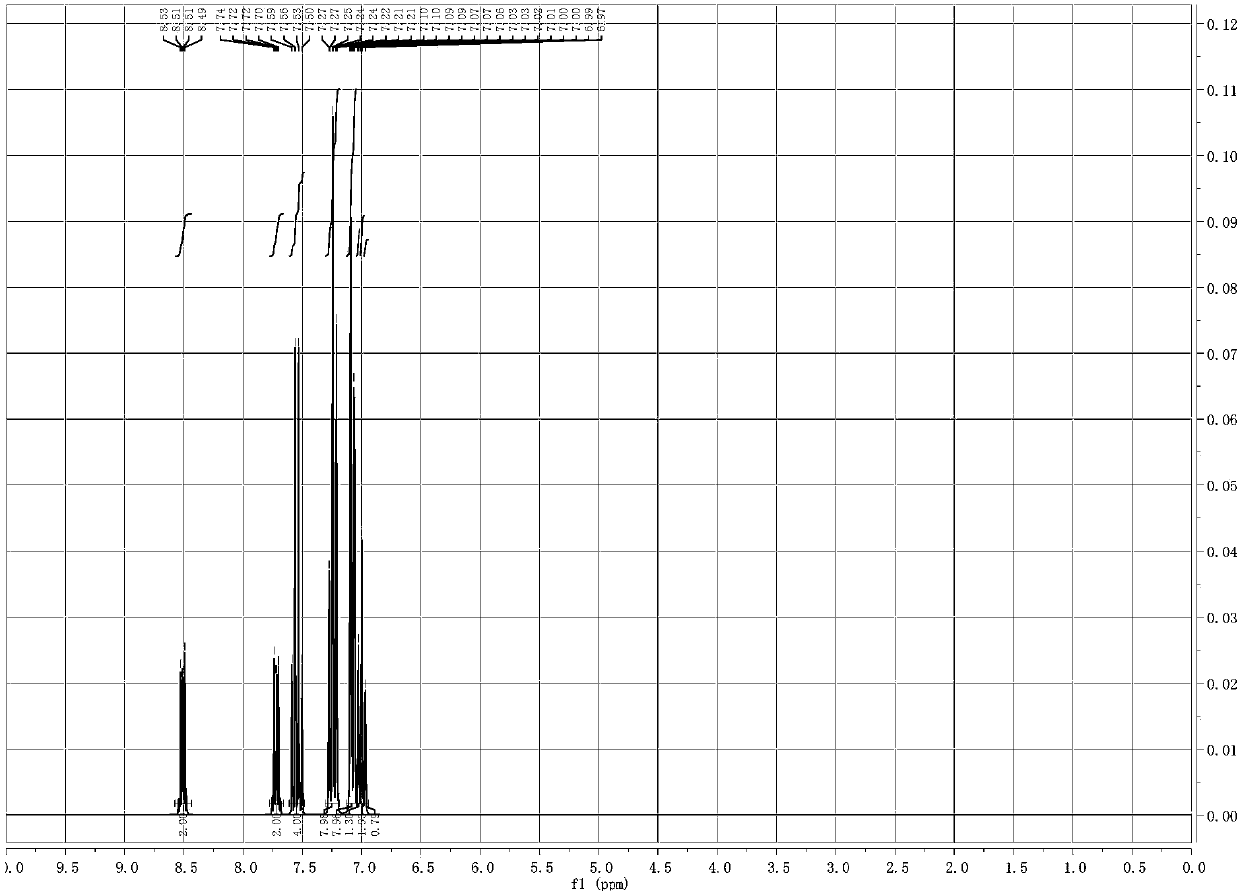
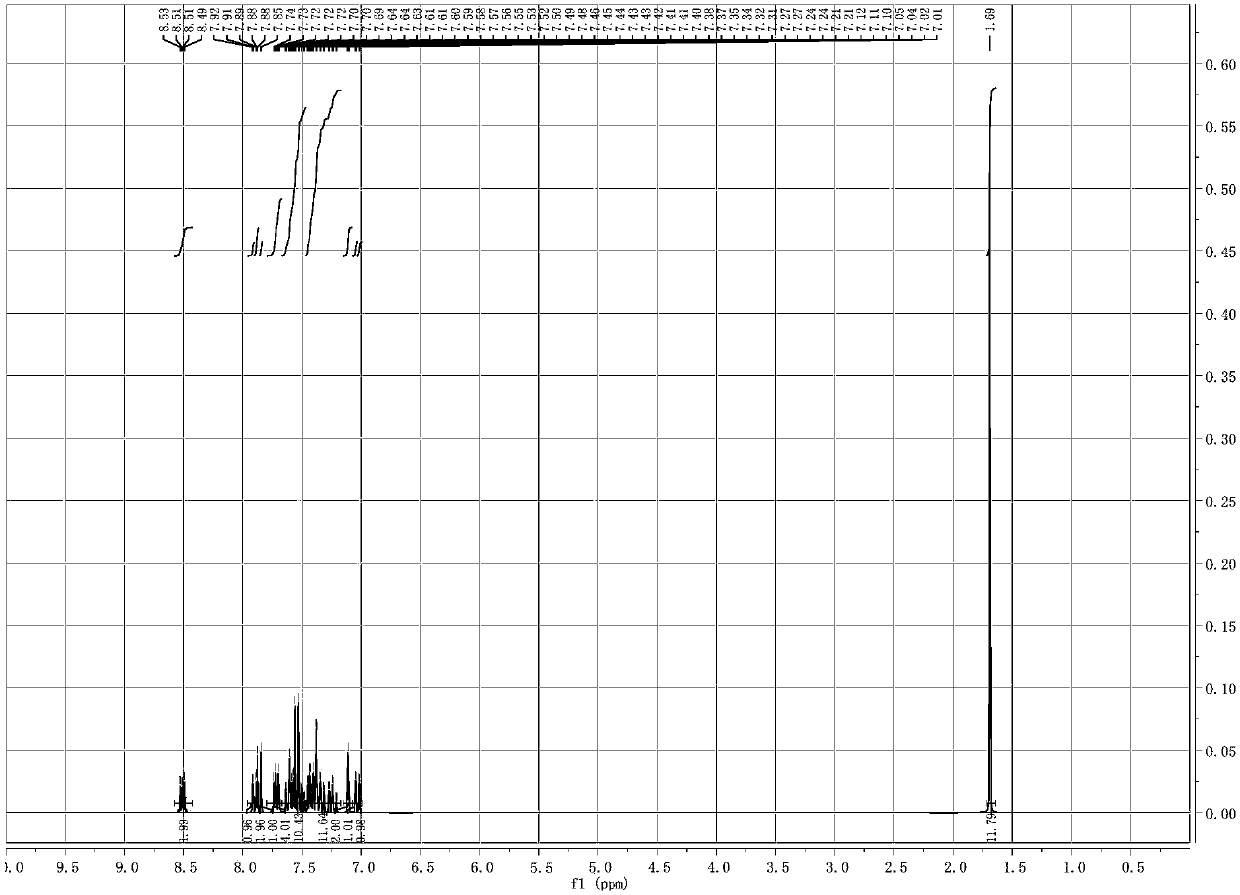
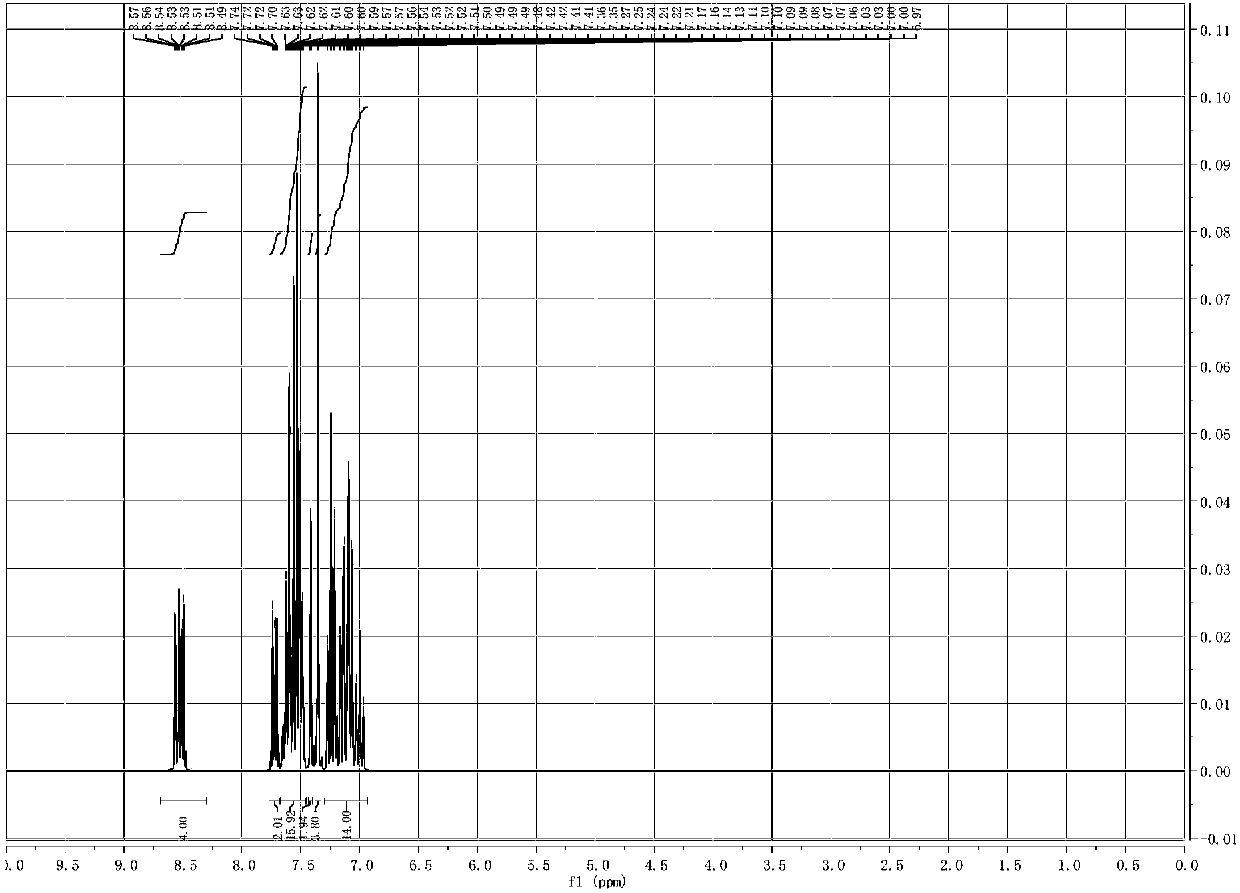
Image
Examples
preparation example Construction
[0060] Synthetic Example 1. The preparation of intermediate M1, its synthetic route is as follows:
[0061]
[0062] 1,4-Diaminonaphthalene (20mmol, 3.16g) and triethylamine (60mmol, 6.06g) were mixed and dissolved in 50ml of dichloromethane, lowered to 0°C, and 3- Ethoxyacryloyl chloride (50mmol, 6.7g), after the dropwise addition, was slowly warmed up to room temperature and stirred, the reaction end was monitored by TLC, and the reaction was completed in 5 hours. Quenched with water, extracted with dichloromethane, the extract was concentrated, passed through a silica gel column, the eluent was petroleum ether:dichloromethane=8:1, and the eluent was concentrated to obtain intermediate M1-A (6.44g, yield 91%).
[0063] Intermediate M1-A (17mmol, 6g) was dissolved in 60mL of dichloromethane, the mixture was lowered to 0°C, methanesulfonic acid (51mmol, 4.9g) was added dropwise with a constant pressure dropping funnel, and after the addition was completed, it was raised to...
Embodiment 3
[0071] Embodiment 3. The synthesis of compound A2, the synthetic route is as follows:
[0072]
[0073] Step 1: Synthesis of M2
[0074] Under nitrogen protection, add 2,4-dimethylaniline 2.67g (molecular weight 121,
[0075] 22mmol), 2,4-dimethylbromobenzene 3.68g (molecular weight 184, 20mmol), sodium tert-butoxide 5.76g (molecular weight 96, 60mmol), Pd2(dba) 3 183mg (molecular weight 916, 0.2mmol), P( tBu)3 121 mg (molecular weight 202, 0.6 mmol). Anhydrous toluene 50ml. After the addition was completed, the oil bath was slowly heated to reflux for reaction, and the end point of the reaction was monitored by TLC, and the reaction was completed in 10 hours. Cool, add water to quench the reaction, extract with 50ml of dichloromethane, the organic phase is dried with anhydrous MgSO4, the organic phase is evaporated to dryness, and the obtained solid is separated by column chromatography to obtain 3.8g white solid, molecular weight 225, yield 85%.
[0076] The second st...
Embodiment 4
[0078] Embodiment 4. The synthesis of compound A3, the synthetic route is as follows:
[0079]
[0080] Step 1: Synthesis of M3
[0081] The method is the same as the synthesis of M2 in Example 3, except that 2,4-dimethylaniline and 2,4-dimethylbromobenzene are replaced by 4-cyclohexylaniline and 4-cyclohexylbromobenzene respectively, and separated by column chromatography to obtain 5.5 g of white product, yield 83%.
[0082] The second step: the implementation process is the same as in Example 2, except that diphenylamine is replaced by intermediate M3 to synthesize the product.
[0083] Product MS (m / e): 892, elemental analysis (C62H68N4): theoretical value C: 86.05%, H: 7.67%, N: 6.27%; found value C: 86.00%, H: 7.63%, N: 6.37%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com