Ferrum-base block non-crystalline alloy material
A technology of amorphous alloys and large blocks, which is applied in the field of amorphous alloys, can solve the problems of increased difficulty of amorphous alloys, relatively high requirements for raw material purity, and increased raw material prices, and achieve good amorphous formation capabilities, low cost, and The effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
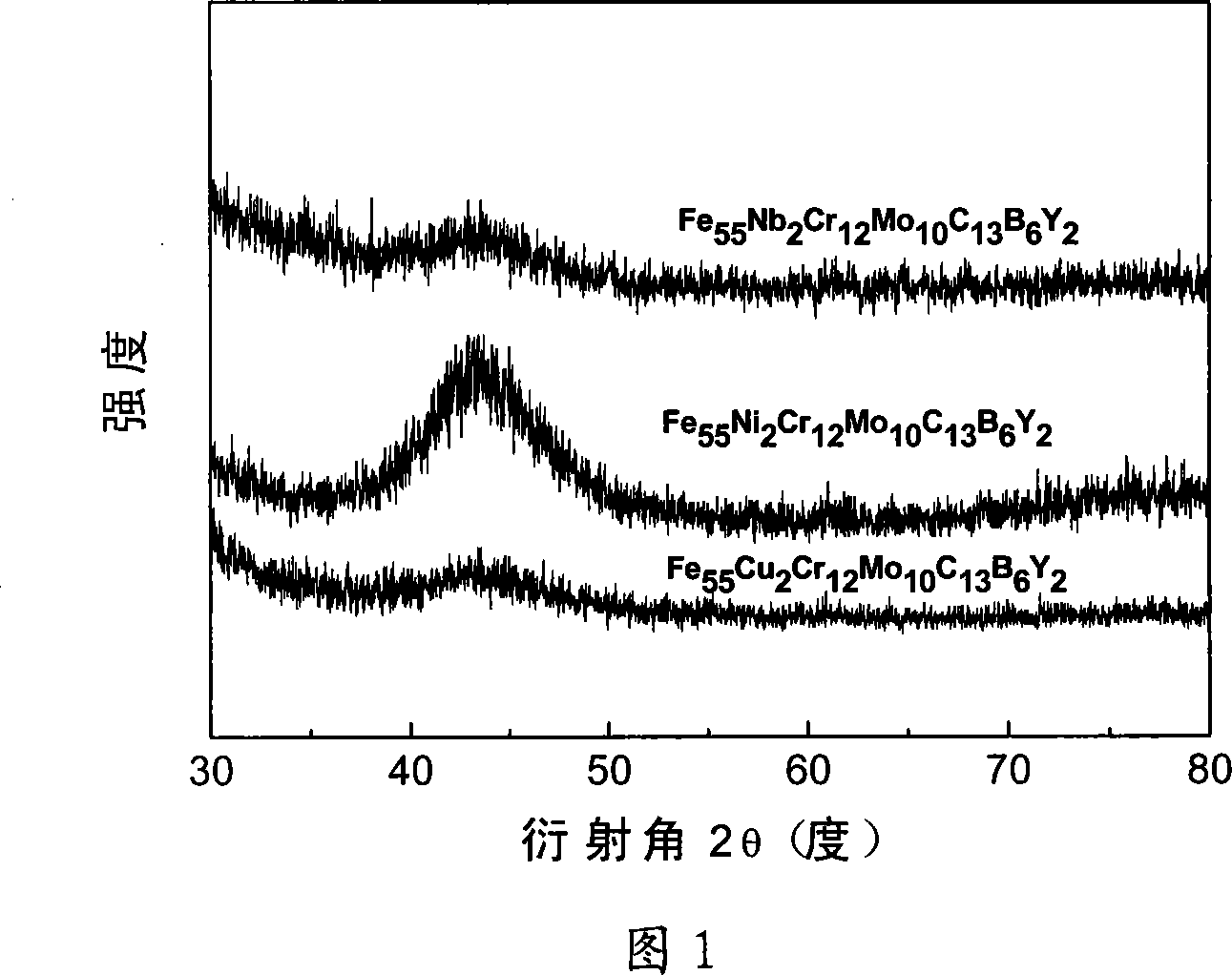
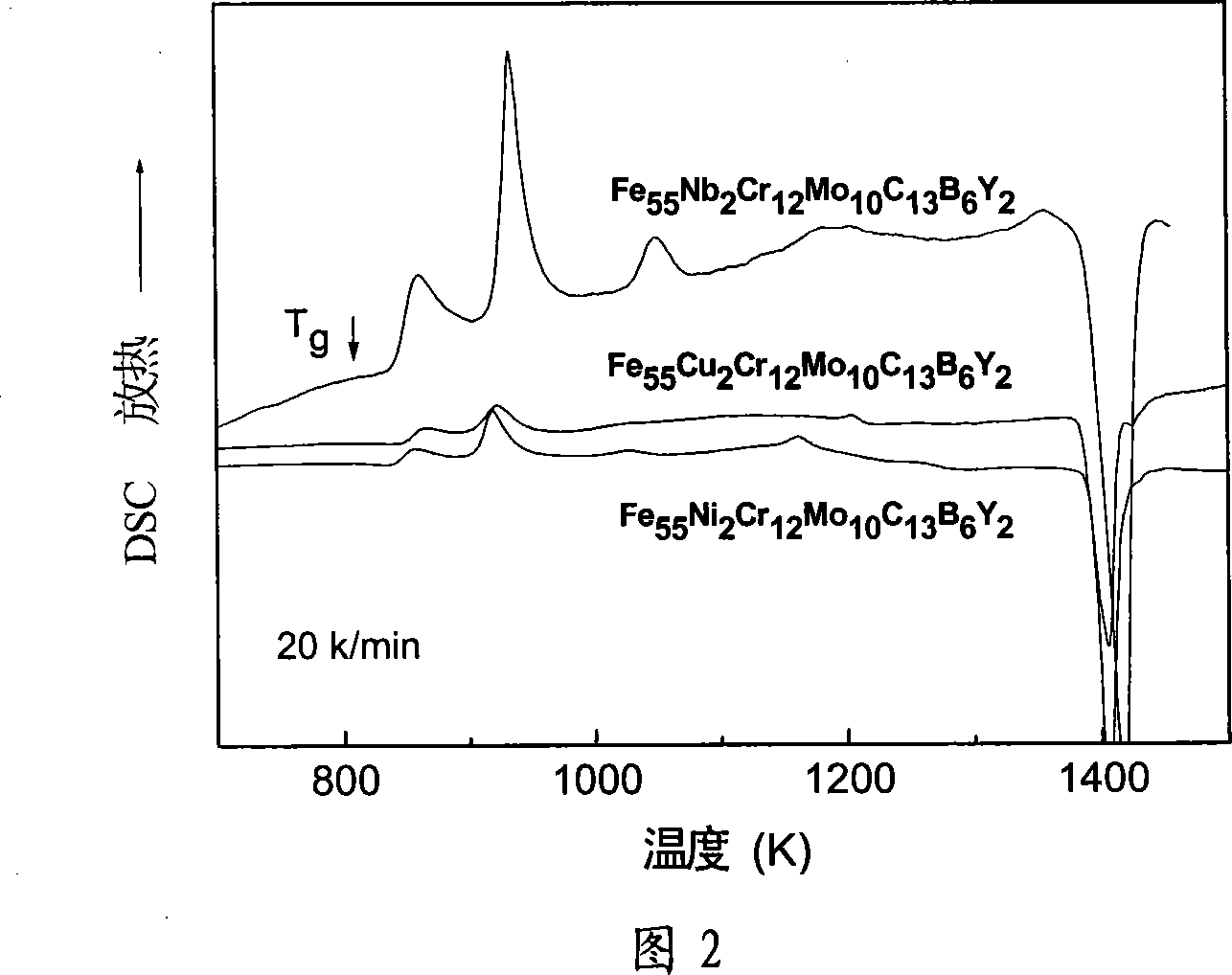
[0030] The industrially used pure metals Fe, Ni, Cr, Mo, Y, C and the industrially used Fe-B alloy are prepared according to the required atomic percentage as raw materials, and are arc melted in an argon protective atmosphere where zirconium absorbs oxygen. The electric arc furnace is equipped with suction casting equipment. After remelting the smelted alloy, suction casting is carried out. After the alloy is injected into the copper mold, Fe with uniform composition is formed. 55 Ni 2 Cr 12 Mo 10 C 13 B 6 Y 2 Large piece of amorphous alloy with a diameter of 4mm. The XRD diffraction pattern proves that the alloy is an amorphous alloy. From the DSC curve, it can be measured that the glass transition temperature (Tg) of the amorphous alloy is 809K, and the initial crystallization temperature (Tg) x1 ) is 841K, melting point (T m ) is 1392K, liquidus temperature (T 1 ) is 1425K, the width of the supercooled liquid phase region (ΔT x ) is 32K, the parameter (γ) is 0.37...
Embodiment 2
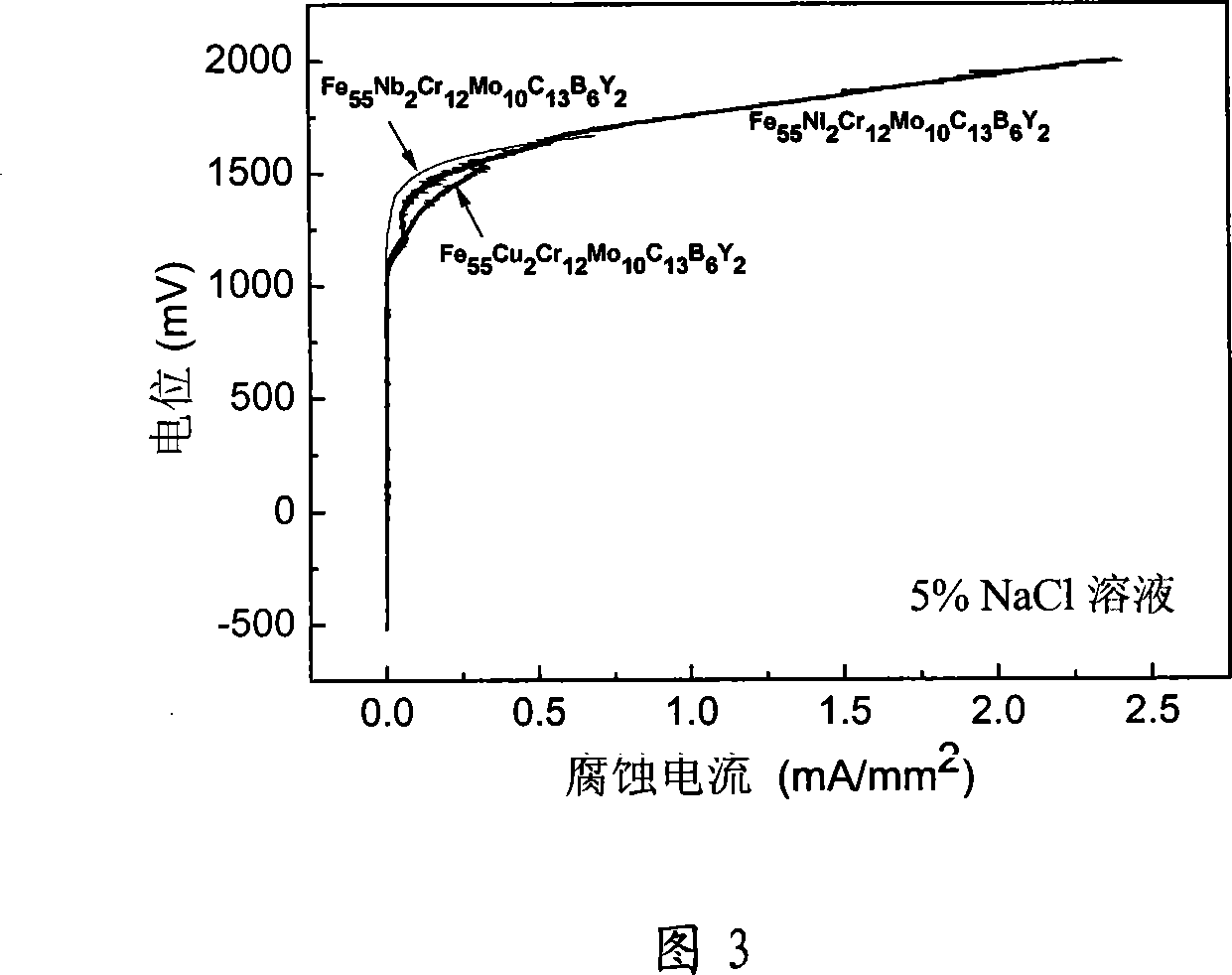
[0032] Technical scheme is as embodiment 1, preparation composition is Fe 55 Cu 2 Cr 12 Mo 10 C 13 B 6 Y 2 A bulk amorphous alloy obtained by replacing Ni in the alloy with Cu. Using the technical scheme described in Example 1, an amorphous cylindrical rod with a diameter of 4 mm can be prepared. The glass transition temperature of the alloy is 815K, the initial crystallization temperature is 848K, the melting point is 1385K, and the liquidus temperature (T 1 ) is 1436K, the width of the supercooled liquid phase region (ΔT x ) is 33K, the parameter (γ) is 0.3767, the approximate glass transition temperature is 0.568, and the Vickers hardness is 1253. The passivation potential of the alloy also reached 1.5V in 5% NaCl solution and 1mol / L HCl corrosion solution. The passivation current densities in NaCl solution and HCl solution were 2×10 -3 mA / mm 2 and 5×10 -3 mA / mm 2 . Than Fe 55 Ni 2 Cr 12 Mo 10 C 13 B 6 Y 2 The passivation current density of amorphous al...
Embodiment 3
[0034] Technical scheme is as embodiment 1, uses the Fe-Nb alloy preparation composition of industrial application to be Fe 55 Nb 2 Cr 12 Mo 10 C 13 B 6 Y 2 A bulk amorphous alloy obtained by replacing Ni in the alloy with Nb. Using the technical scheme described in Example 1, an amorphous cylindrical rod with a diameter of 2 mm can be prepared. The glass transition temperature of the alloy is 794K, the initial crystallization temperature is 841K, the melting point is 1390K, and the Vickers hardness is 1325. The temperature of the supercooled liquid phase region of this alloy is 47K, and the liquid phase temperature (T 1 ) is 1422K, and the parameter (γ) is 0.3795, but the approximate glass transition temperature is 0.558. Considering that the amorphous formation ability of this alloy is not as good as that of Fe 55 Ni 2 Cr 12 Mo 10 C 13 B 6 Y 2 and Fe 55 Cu 2 Cr 12 Mo 10 C 13 B 6 Y 2 The alloy has strong amorphous forming ability. The passivation potenti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| Vickers hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com