Preparation method for extracting purified general saponin from notoginseng of Chinese traditional medicine
A technology for total saponins and Panax notoginseng, applied in the field of medicine, can solve the problems of low content of controllable components, influence on the safety and effectiveness of Panax notoginseng saponins, and disadvantages, and achieves low cost, easy industrialized production, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
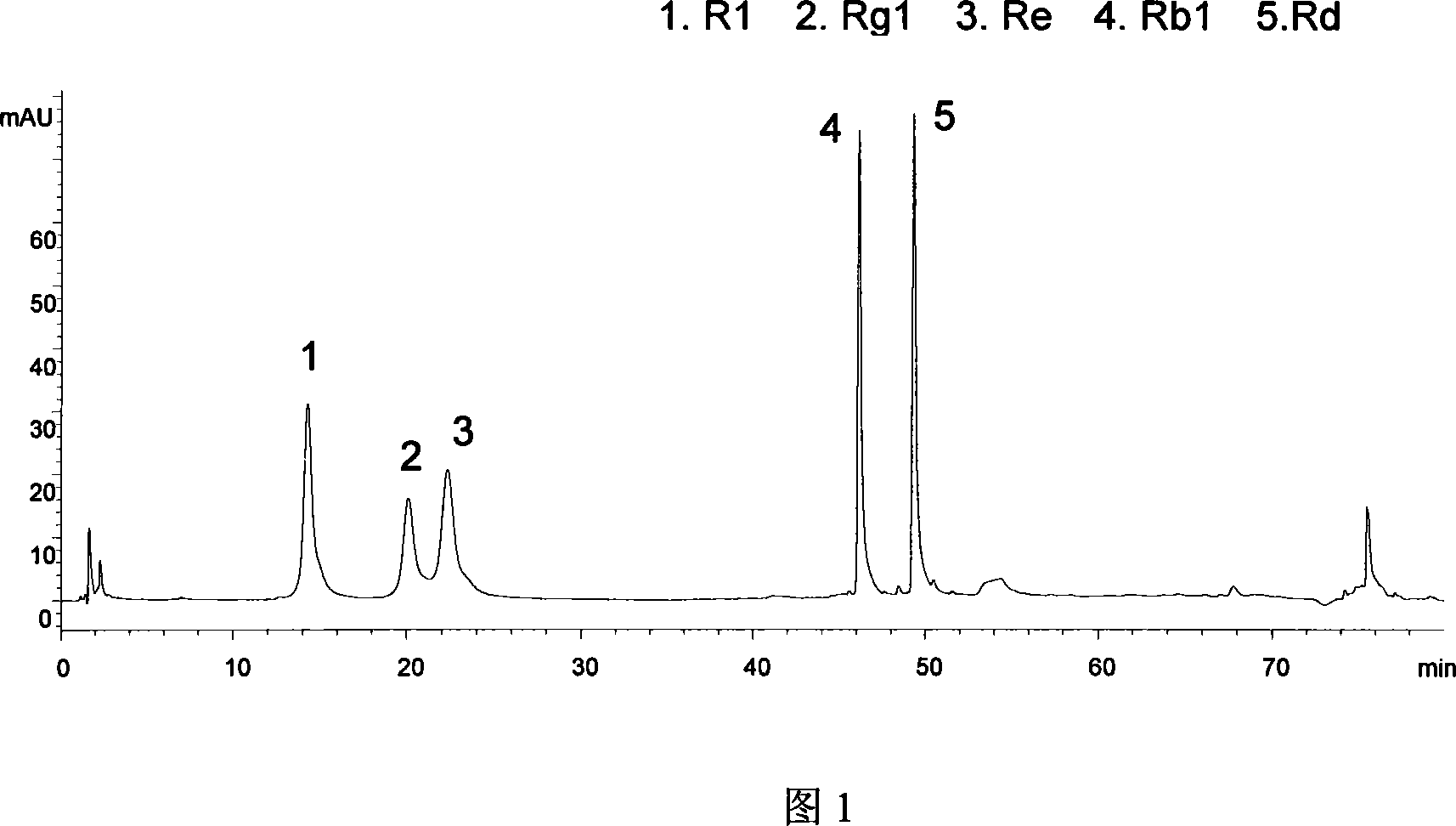
[0020] Take 500g of the coarse particle of Panax notoginseng (particle diameter about 1cm), dry at 50°C for 6 hours, add 8 times the amount of 30% ethanol and heat it under reflux to extract twice, 1 hour each time, filter the extract, combine, and concentrate under reduced pressure To 0.5g / mL (crude drug amount), go to D 101 Macroporous resin. Rinse with 4 times the column volume of deionized water, discard, continue to rinse with 4BV 30% ethanol solution, discard, and finally elute with 5BV 70% ethanol solution, collect the eluate, concentrate under reduced pressure to no alcohol, freeze-dry , that is, Panax notoginseng total saponins. As determined by HPLC, the notoginseng saponin R in the refined 1 2%, Ginsenoside Rg 1 15%, Ginsenoside Rb 1 9%, ginsenoside Rd 3%, ginsenoside Re 1%, the total saponin content reaches 30%.
Embodiment 2
[0022] Take 500 g of the coarse particle (particle diameter of about 1 cm) of the medicinal material of notoginseng, dry it at 50°C for 6 hours, add 15 times the amount of 40% ethanol and heat it under reflux for extraction once in a slightly boiling state, for 2 hours, filter the extract, combine it, and concentrate it under reduced pressure to 0.5 g / mL (crude drug amount), above D 101 Macroporous resin. Rinse with 5 times the column volume of deionized water, discard, then rinse with 4BV of 1% ammonia water, discard, then rinse with deionized water until the pH of the effluent = 7, and finally elute with 6BV of 30% ethanol solution, collect the washed Remove liquid, concentrate under reduced pressure to no alcohol, and freeze-dry to obtain total saponins of Panax notoginseng. As determined by HPLC, the notoginseng saponin R in the refined1 4%, Ginsenoside Rg 1 30%, Ginsenoside Rb 1 15%, ginsenoside Rd 5%, ginsenoside Re 3%, the total saponin content reaches 57%.
Embodiment 3
[0024] Take 500g of the coarse particle of Panax notoginseng medicinal material (particle diameter about 1cm), dry at 50°C for 6 hours, add 18 times the amount of 50% ethanol and heat and reflux in a slightly boiling state to extract once for 1 hour, filter the extract, combine, and concentrate under reduced pressure to 0.5 g / mL (crude drug amount), above D 101 Macroporous resin. Rinse with 4 times the column volume of deionized water, discard, then rinse with 4BV of 0.1% ammonia water, discard, then rinse with deionized water until the pH of the effluent = 7, and finally elute with 5BV of 60% ethanol solution, collect the washed Remove liquid, concentrate under reduced pressure to no alcohol, and freeze-dry to obtain total saponins of Panax notoginseng. As determined by HPLC, the notoginseng saponin R in the refined 1 4%, Ginsenoside Rg 1 26%, Ginsenoside Rb 1 16%, ginsenoside Rd 5%, ginsenoside Re 4%, the total saponin content reaches 55%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com