Carrier of liposome medication, and preparation method
A liposome and drug technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problem that liposomes are not easy to sterilize, cannot guarantee the stability of drugs, liposomes Problems such as difficulty in ultrafiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of spontaneous liposomes
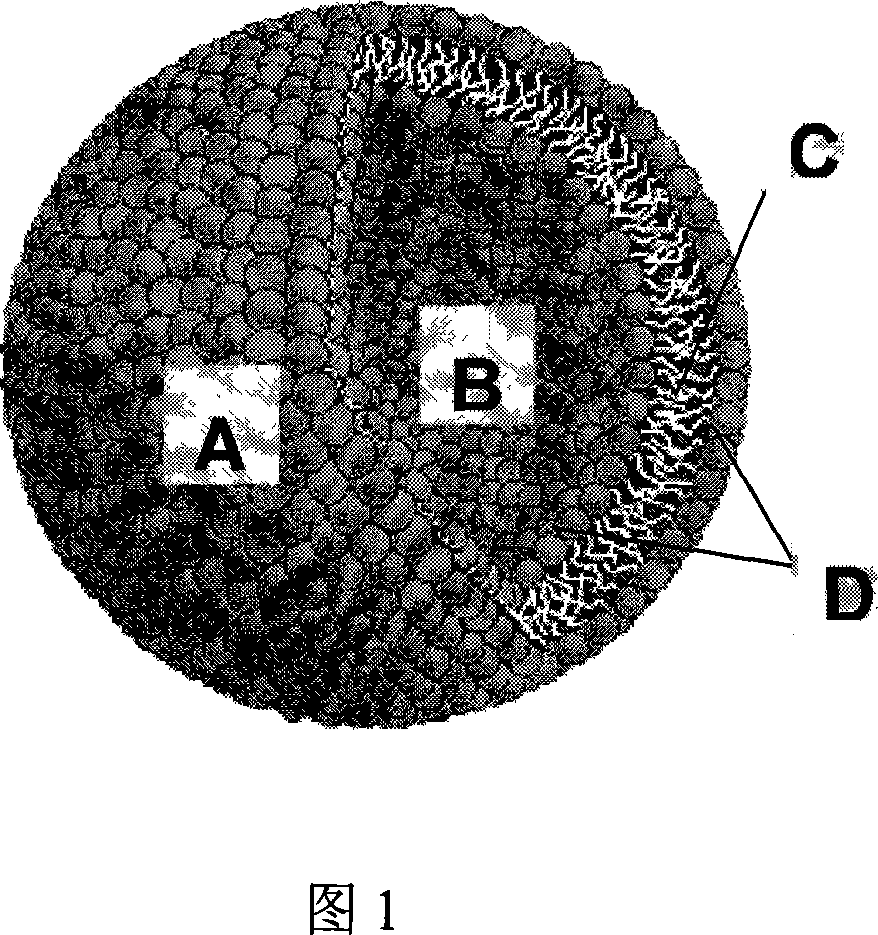
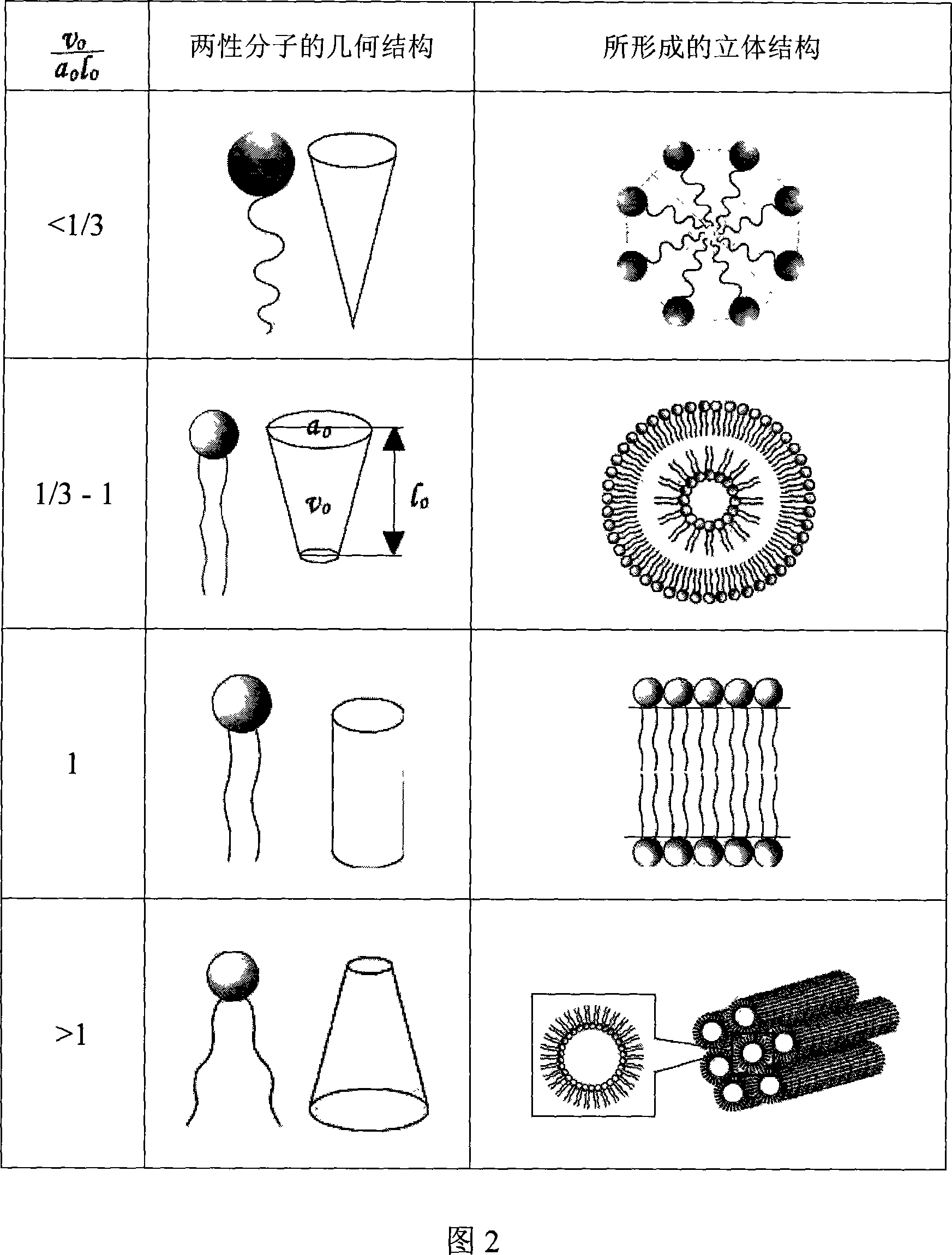
[0044] The main component of the liposome, polyethylene glycol diglyceride, will be described by taking polyethylene glycol diglyceride as an example. Liposomes form spontaneously when polyethylene glycol diolein is mixed with an aqueous solution. Polyethylene glycol 200, 400, 600, 800 and 1500 glyceryl dioleate was synthesized from DuPont R&D Center (Wimington, Delaware, USA), and the water was purified water. The lipid and water were preheated to the design temperature (see Table 1), and then various polyethylene glycol diolein lipids were slowly added to the stirred aqueous solution, and then stirred for two hours or until the equilibrium was reached. At room temperature, the formation of liposomes was observed with optical microscope and electron microscope respectively. The final concentration of polyethylene glycol diolein was 10%. At room temperature (20° C.), polyethylene glycol 200, 400, and 600 diolein all spontaneously...
Embodiment 2
[0049] Preparation of Spontaneous Liposomes of Polyethylene Glycol Diacylglycerides with Different Fatty Acid Chains
[0050] To illustrate that other polyethylene glycol diglycerides also spontaneously form liposomes, stearic acid (stearic acid), palmitic acid (palmitic acid), myristic acid (myristic acid), lauric acid (dodecanoic acid) and their respective combinations with oleic acid (octadecenoic acid), using polyethylene glycol 600 as an example to synthesize polyethylene glycol diglyceride (provided by the DuPont R&D Center in the United States), to observe its Case of spontaneous liposome formation. The synthetic lipids and purified water were equilibrated to the designed temperature, and then the lipids were slowly added to the stirring water, and then stirred for two hours or until the equilibrium reached room temperature, and the liposomes were observed with an optical microscope and an electron microscope, respectively. Formation. The final concentration of polyet...
Embodiment 3
[0053] Phospholipid spontaneous liposomes
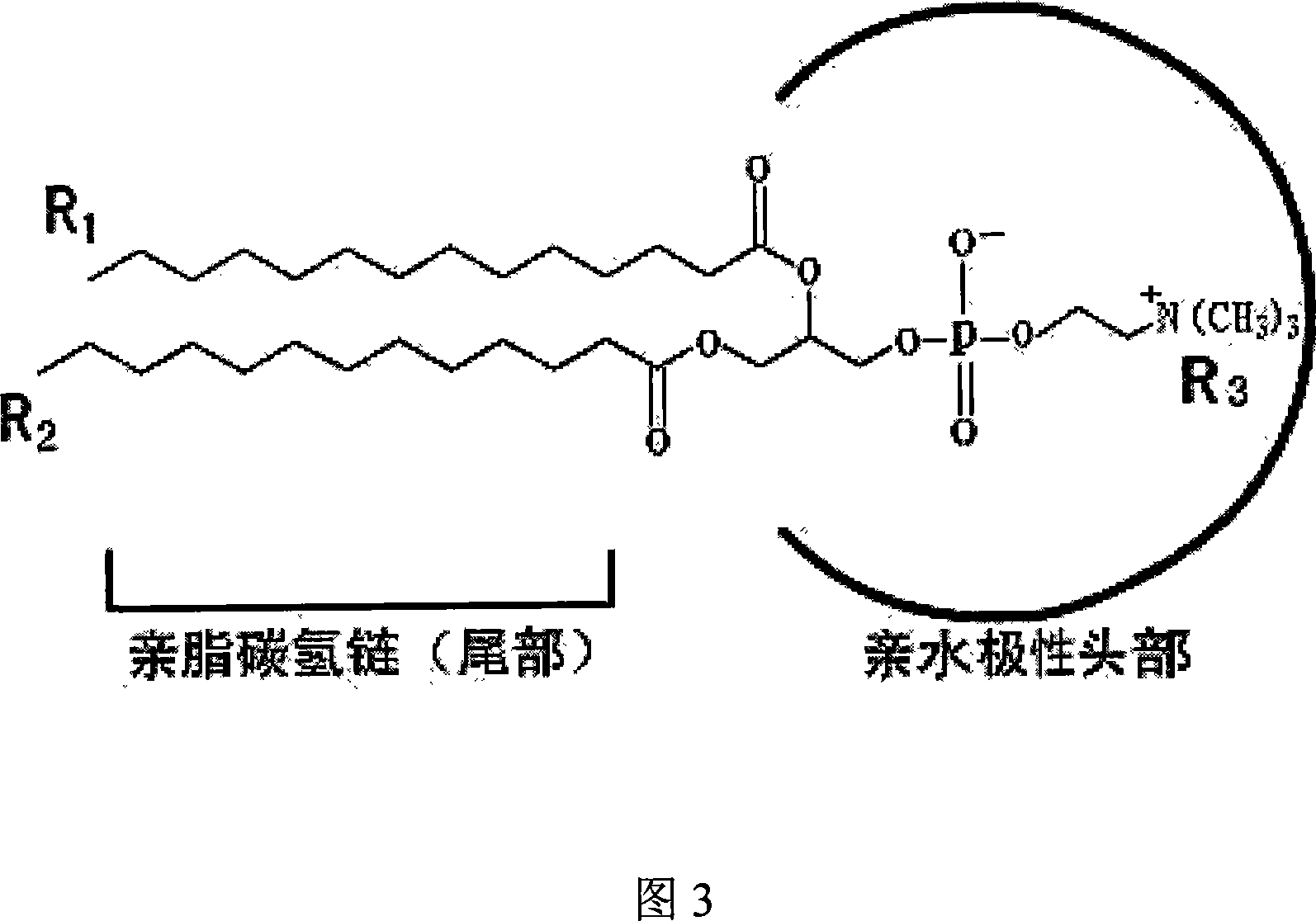
[0054] Take polyethylene glycol 600 glyceryl diolein as an example, mix with different concentrations of phosphates to verify the effect of phosphates on the formation of spontaneous liposomes of polyethylene glycol diolein, and the phosphates are POPC( 1-palmitoyl, 2-oleoylphosphorylcholine) were purchased from Sigma, and water was purified water. The ratios of POPC to polyethylene glycol 600 diolein were 5%, 10%, 15%, 20%, 30%, and 50%, respectively. POPC and Polyethylene Glycol 600 Diolein were first stirred and mixed evenly in proportion, and the mixed lipid and water were preheated to the required temperature (room temperature, 37°C and 50°C, Table 3), and then the mixed fat was slowly Slowly add to the stirring water, continue to stir for two hours or until room temperature, take a sample under the light microscope and electron microscope to observe the formation of liposomes, the final lipid concentration in this experiment i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com