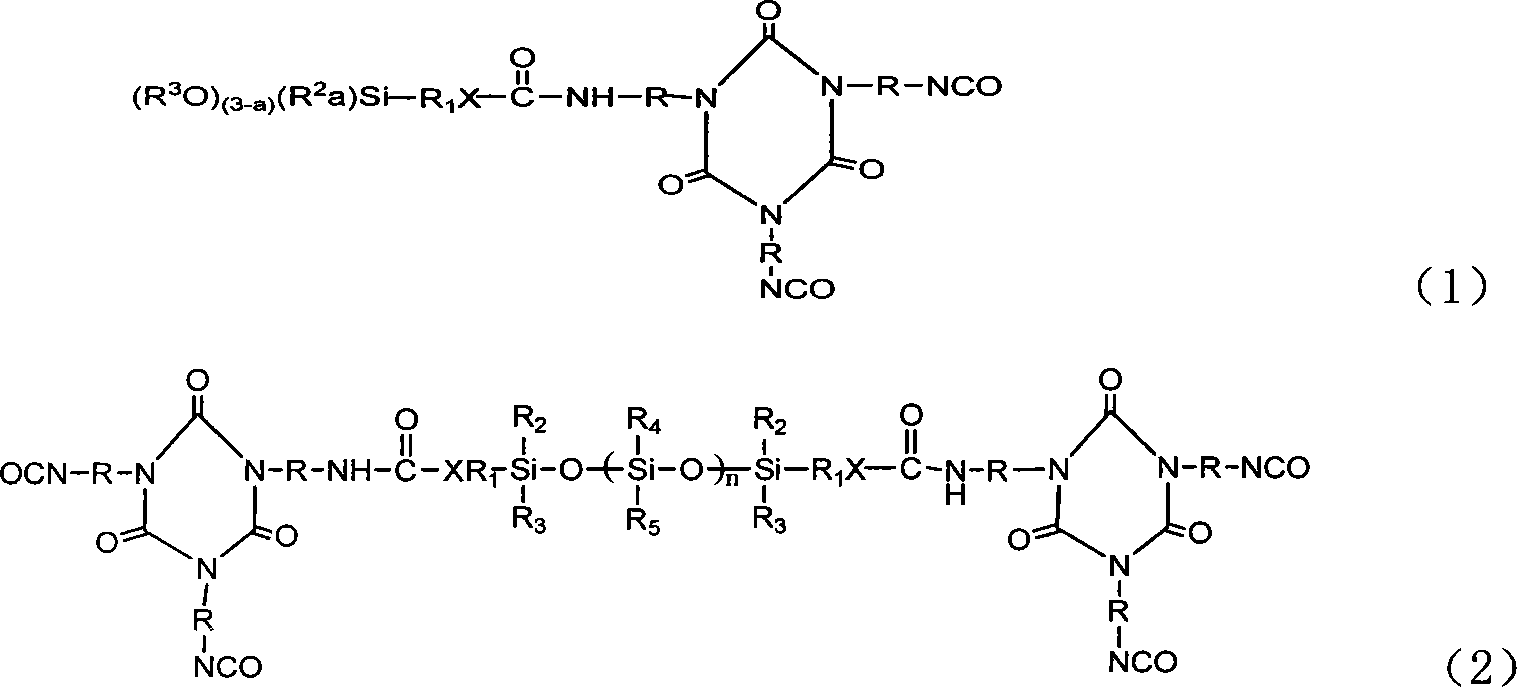
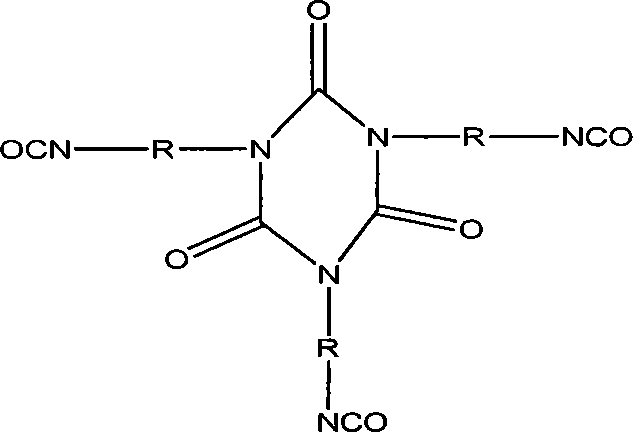
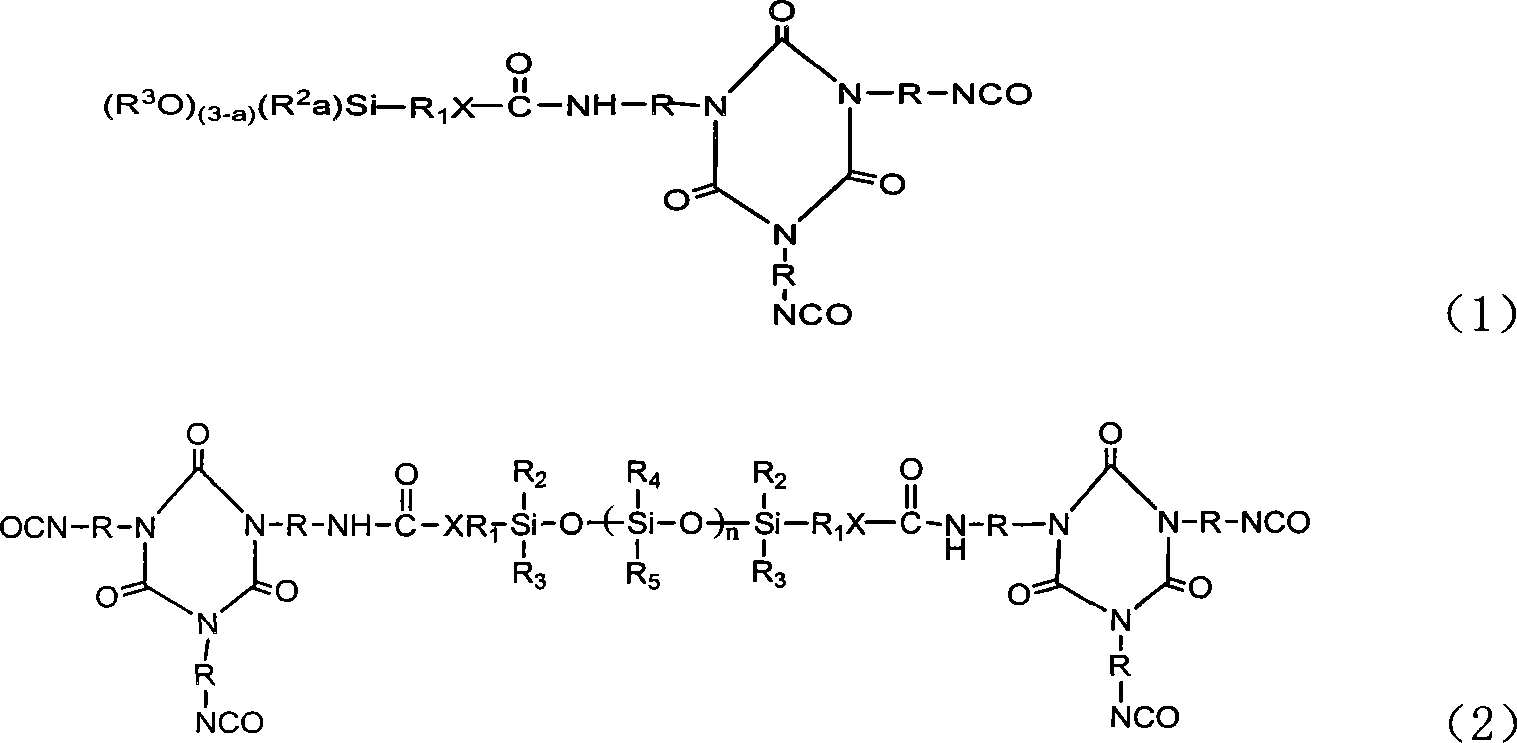
Tripolymer in isocyanic ester class modified by silicane or functional polusiloxane, preparation method
A technology of isocyanate trimerization and polysiloxane, which is applied in chemical instruments and methods, polyurea/polyurethane coatings, compounds of elements of Group 4/14 of the periodic table, etc., can solve the problem of less silicone modified isocyanate trimers. and other problems, to achieve the effect of good flexibility, good color retention and gloss retention, and improved tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] [Example 1] Compound A:
[0031] Dissolve 52.2 grams of TDI trimer in 400 ml of anhydrous tetrahydrofuran, slowly dropwise add 22.1 grams of γ-aminopropyltriethoxysilane and 0.07 grams of dibutyltin dilaurate, and keep at room temperature Under a dry environment, the reaction was stirred for 5 hours; after the reaction was completed, the solvent anhydrous tetrahydrofuran was removed under reduced pressure to obtain 68.36 g of product A of the following chemical formula with a yield of 92%.
[0032]
[0033] Elemental Analysis C 36 h 41 N 7 o 9 Si found (calculated) / %: C 58.02 (58.14), H 5.43 (5.52), N 13.07 (13.19), O 19.29 (19.38), Si 3.69 (3.77).
[0034] 1 H NMR (δ / ppm): 0.58(t, 2H), 1.22(q, 3H), 1.6(q, 2H), 2.0(m, 2H), 2.35(m, 3H), 2.65(q, 2H), 3.83 (q, 2H), 6.9 (t, 1H), 7.0 (q, 1H), 7.26 (m, 1H), 7.5 (m, 2H), 7.9 (s, 1H).
Embodiment 2
[0035] [Example 2] Compound B:
[0036] The TDI trimer of 52.2 grams is dissolved in the anhydrous tetrahydrofuran of 400ml, slowly adds in the two [3-(triethoxysilyl) propyl group] amine of 44 grams and 0.07 gram dibutyltin dilaurate , at room temperature and kept in a dry environment, stirred and reacted for 5 hours; after the reaction was completed, the solvent anhydrous tetrahydrofuran was removed under reduced pressure to obtain 80.5 grams of product B of the following chemical formula, with a yield of 85%.
[0037]
[0038] Elemental Analysis C 45 h 61 N 7 o 12 Si 2 Found (calculated) / %: C 56.89 (57.02), H 6.33 (6.44), N 10.26 (10.35), O 20.16 (20.27), Si 5.79 (5.91).
[0039] 1 H NMR (δ / ppm): 0.58(t, 2H), 1.22(q, 3H), 1.6(q, 2H), 2.0(m, 2H), 2.35(m, 3H), 2.65(q, 2H), 3.83 (q, 2H), 6.9 (t, 1H), 7.0 (q, 1H), 7.26 (m, 1H), 7.5 (m, 2H), 7.9 (s, 1H).
Embodiment 3
[0040] [Example 3] Compound C:
[0041] 52.2 grams of TDI trimer was dissolved in 400ml of anhydrous tetrahydrofuran, slowly added dropwise to 22.2 grams of aminoethylaminopropyltrimethoxysilane and 0.07 grams of dibutyltin dilaurate, at room temperature and kept Under a dry environment, the reaction was stirred for 5 hours; after the reaction was completed, the solvent anhydrous tetrahydrofuran was removed under reduced pressure to obtain 70.68 g of product C of the following chemical formula, with a yield of 95%.
[0042]
[0043] Elemental Analysis C 35 h 40 N 8 o 9 Si found (calculated) / %: C 56.32 (56.45), H 5.26 (5.38), N 14.97 (15.05), O 19.28 (19.35), Si 3.69 (3.76).
[0044] 1 H NMR (δ / ppm): 1.22(q, 3H), 1.6(q, 2H), 2.0(m, 2H), 2.35(m, 3H), 2.65(q, 2H), 3.83(q, 2H), 6.0 (s, 1H), 6.9 (t, 1H), 7.0 (q, 1H), 7.26 (m, 1H), 7.5 (m, 2H), 7.9 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com