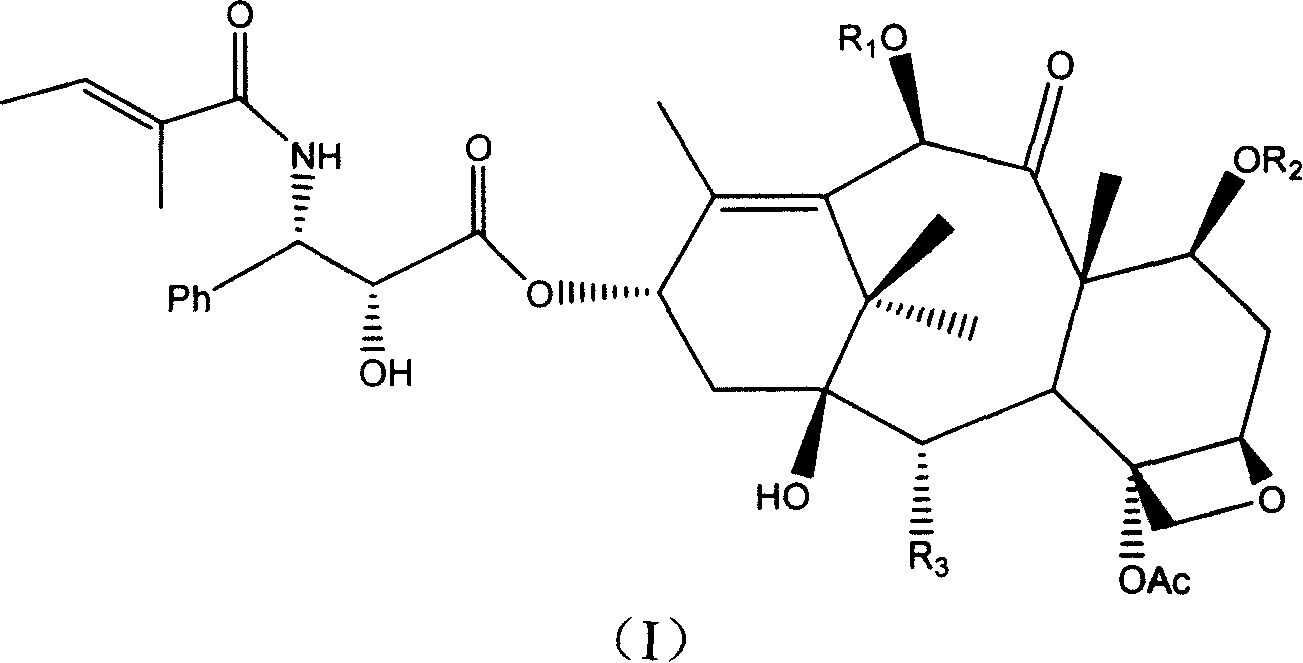
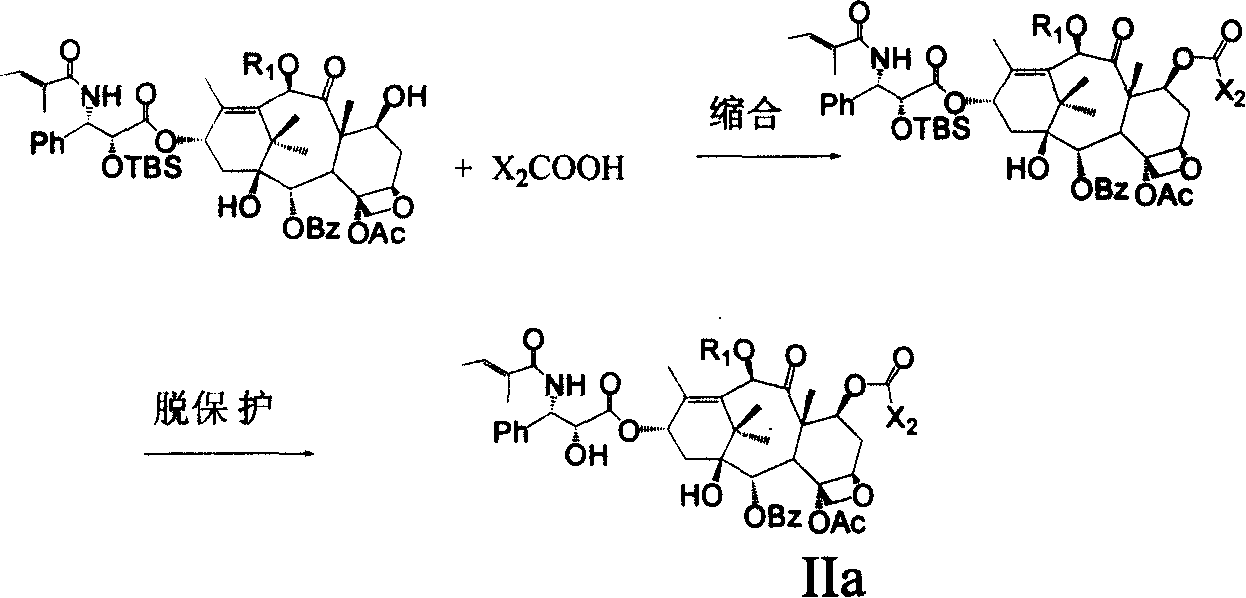
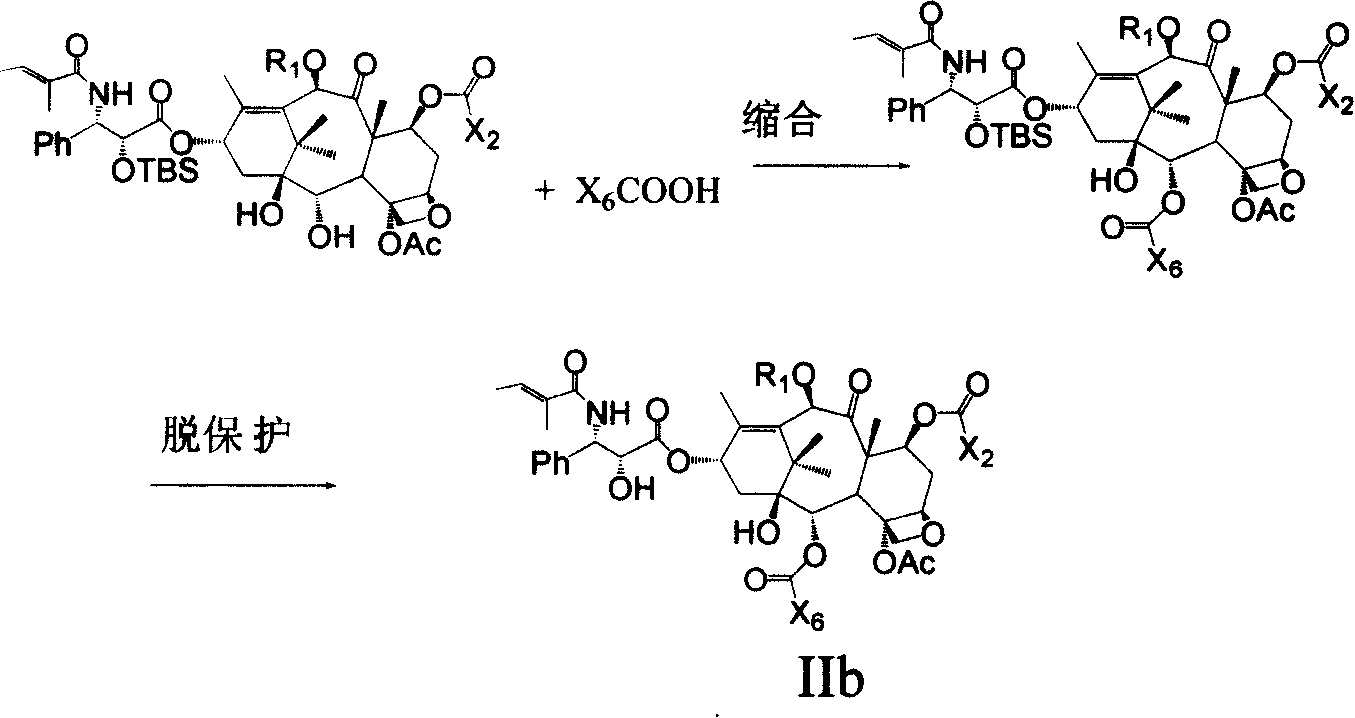
Cephalotamannine derivative, its production, its medicinal composition and use
A technology of cephalomannine and its compounds, applied in the field of cephalomannine derivatives, can solve the problems of high toxicity and side effects, drug resistance, and decline in curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of 10-deacetyl-cephalomanine
[0059]
[0060] Step 1: 2'-tert-butyldimethylsilyl-cephalomannine
[0061] Dissolve cephalomannine (600mg, 0.721mmol) in 5ml of DMF, add imidazole (245.5mg, 3.61mmol) and tert-butyldimethylchlorosilane (543.5mg, 3.61mmol), react at 70°C for 5 hours, add saturated NaHCO 3 10ml of the solution was extracted with ethyl acetate 3×50ml, the ethyl acetate layers were combined, dried over anhydrous sodium sulfate, the ethyl acetate layer was evaporated to dryness, silica gel column chromatography, petroleum ether: ethyl acetate = 5:1. Obtained 637 mg (93.4%) of the target product.
[0062] Step 2: 2'-tert-Butyldimethylsilyl-10-deacetyl-cephalomannine
[0063] Dissolve 2'-tert-butyldimethylsilyl-cephalomannine (98 mg, 0.103 mmol) in 6 ml of ethanol, add 85% hydrazine hydrate (0.625 ml), react at room temperature for 2 hours, add saturated NH 4 10ml of Cl solution was extracted with ethyl acetate 3×50ml, the ethyl aceta...
Embodiment 2
[0069] Example 2 Preparation of 2-(3-azidobenzoyl)-10-propionyl-cephalomannine
[0070]
[0071] Step 1-2: the same as the method described in step 1-2 in Example 1.
[0072] Step 3: 2'-tert-Butyldimethylsilyl-10-propionyl-cephalomannine
[0073] Dissolve 2'-tert-butyldimethylsilyl-10-deacetyl-cephalomannine (155mg, 0.171mmol) in 5mlTHF, add CeCl 3 (8.4mg), ice bath, add propionic anhydride (0.22ml, 1.71mmol), react at 30°C for 2 hours, add 300ml ethyl acetate, wash with saturated NaHCO 3 Solution 2 × 50ml was washed, 50ml saturated NaCl solution was washed, the aqueous layer was extracted with 150ml ethyl acetate, the ethyl acetate layers were combined, dried over anhydrous sodium sulfate, filtered, the ethyl acetate layer was evaporated to dryness, silica gel column chromatography, acetone: Petroleum ether=1:2. 148.2mg (97%) of the target product was obtained.
[0074] Step 4: 2'-tert-Butyldimethylsilyl-7-triethylsilyl-10-propionyl-cephalomannine
[0075] 2'-tert-but...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com