Novel medicine eluting supporting stand
A technology for eluting stents and drugs, used in stents, medical science, surgery, etc., can solve problems such as unexplainable, achieve the effect of reducing burden, good clinical and commercial prospects, and reducing the risk of acute myocardial infarction or death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
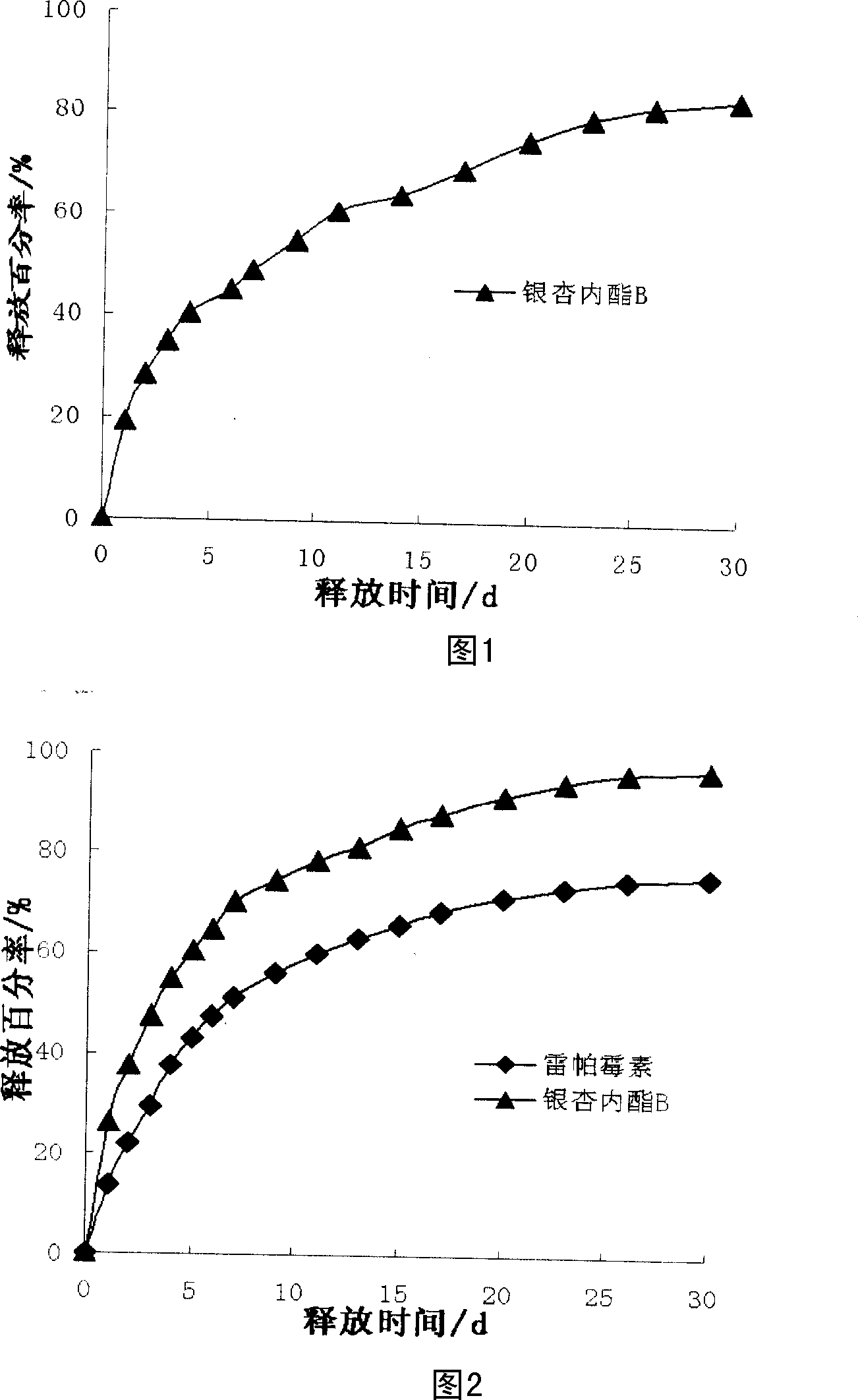
[0034] Weigh an appropriate amount of ginkgolide B and polylactic acid, the weight percentage of which is 3:7, and ultrasonically dissolve them with tetrahydrofuran to obtain a coating solution with a weight percentage of 1%. The prepared coating solution is atomized and sprayed onto the surface of the pretreated bare metal stent. The stent was then dried at 60° C. and a vacuum of 0.1 Mpa to prepare a ginkgolide B coating. Weigh an appropriate amount of polylactic acid, and ultrasonically dissolve it with tetrahydrofuran to obtain a coating solution with a weight percentage of 0.5%. The polylactic acid coating liquid is coated on the surface of the drug stent coated with the ginkgolide B coating by the aforementioned coating method to obtain the ginkgolide B eluting stent.
Embodiment 2
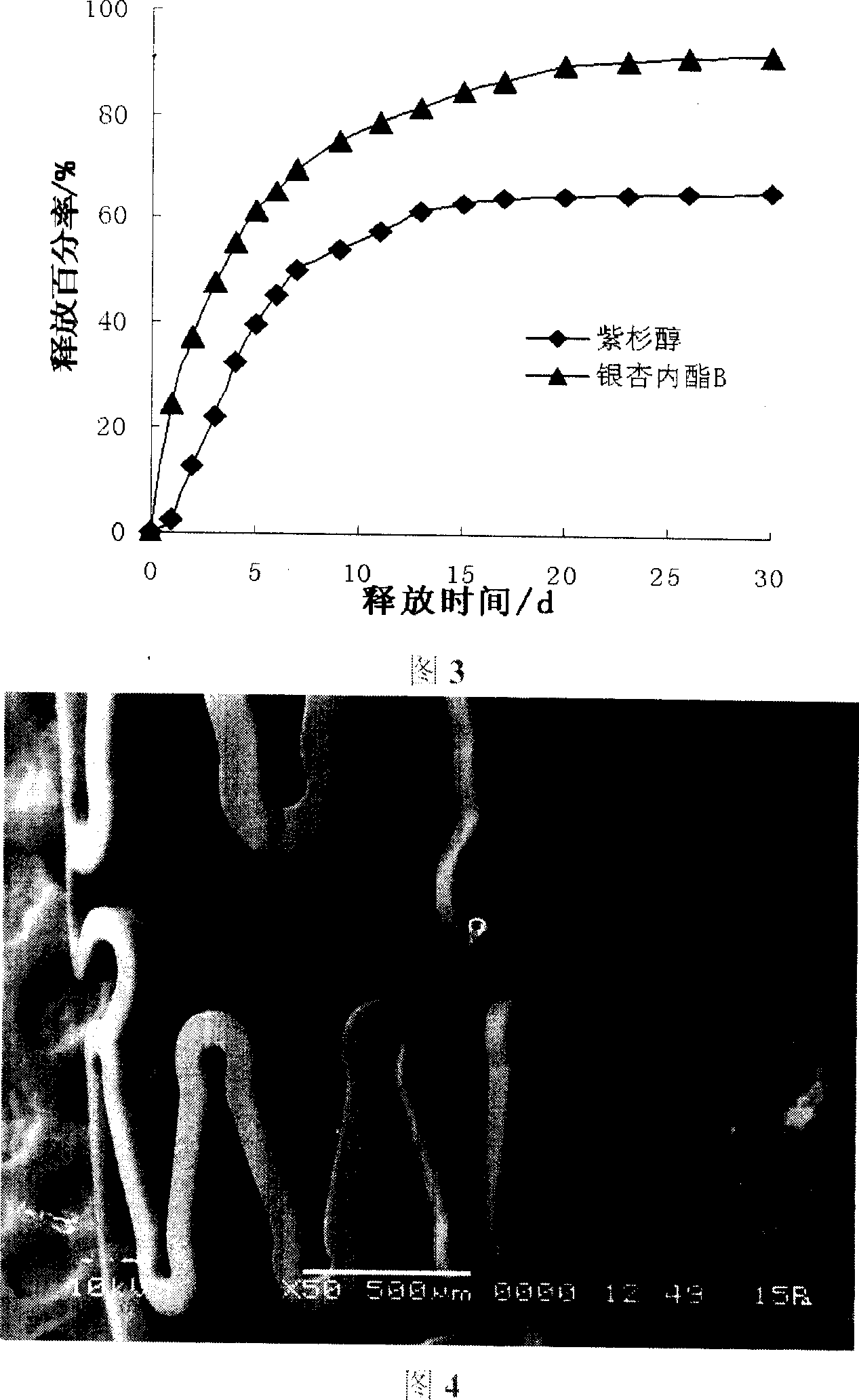
[0036] Weigh an appropriate amount of rapamycin and polymethyl methacrylate nano-silicon dioxide composite material, the weight percentage of the two is 3:7, and ultrasonically dissolve it with tetrahydrofuran to obtain a coating solution with a weight percentage of 1%. The prepared coating solution is atomized and sprayed onto the surface of the pretreated bare metal stent. Then the stent was dried at 60° C. and a vacuum of 0.1 Mpa to prepare a rapamycin coating. Weigh an appropriate amount of ginkgolide B and polymethyl methacrylate nano-silicon dioxide composite material, the weight percentage of the two is 2:8, and ultrasonically dissolve with dichloromethane to obtain a coating solution with a weight percentage of 1%. The ginkgolide B coating liquid is coated on the surface of the rapamycin-coated drug stent by using the aforementioned coating method to obtain a rapamycin-gingkolide B composite eluting stent.
Embodiment 3
[0038] Weigh an appropriate amount of paclitaxel and poly-L-lactic acid (PLLA) with a weight percentage of 4:6, and ultrasonically dissolve them with tetrahydrofuran to obtain a coating solution with a weight percentage of 1%. The prepared coating solution is atomized and sprayed onto the surface of the pretreated bare metal stent. Then the stent was dried at 60° C. and a vacuum of 0.1 Mpa to prepare paclitaxel coating. Weigh an appropriate amount of ginkgolide B and PLLA, the weight percentage of which is 2:8, and ultrasonically dissolve them with tetrahydrofuran to obtain a coating solution with a weight percentage of 1%. Apply the coating solution of ginkgolide B to the surface of the paclitaxel-coated drug stent by using the aforementioned coating method to obtain a paclitaxel-ginkgolide B composite eluting stent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com