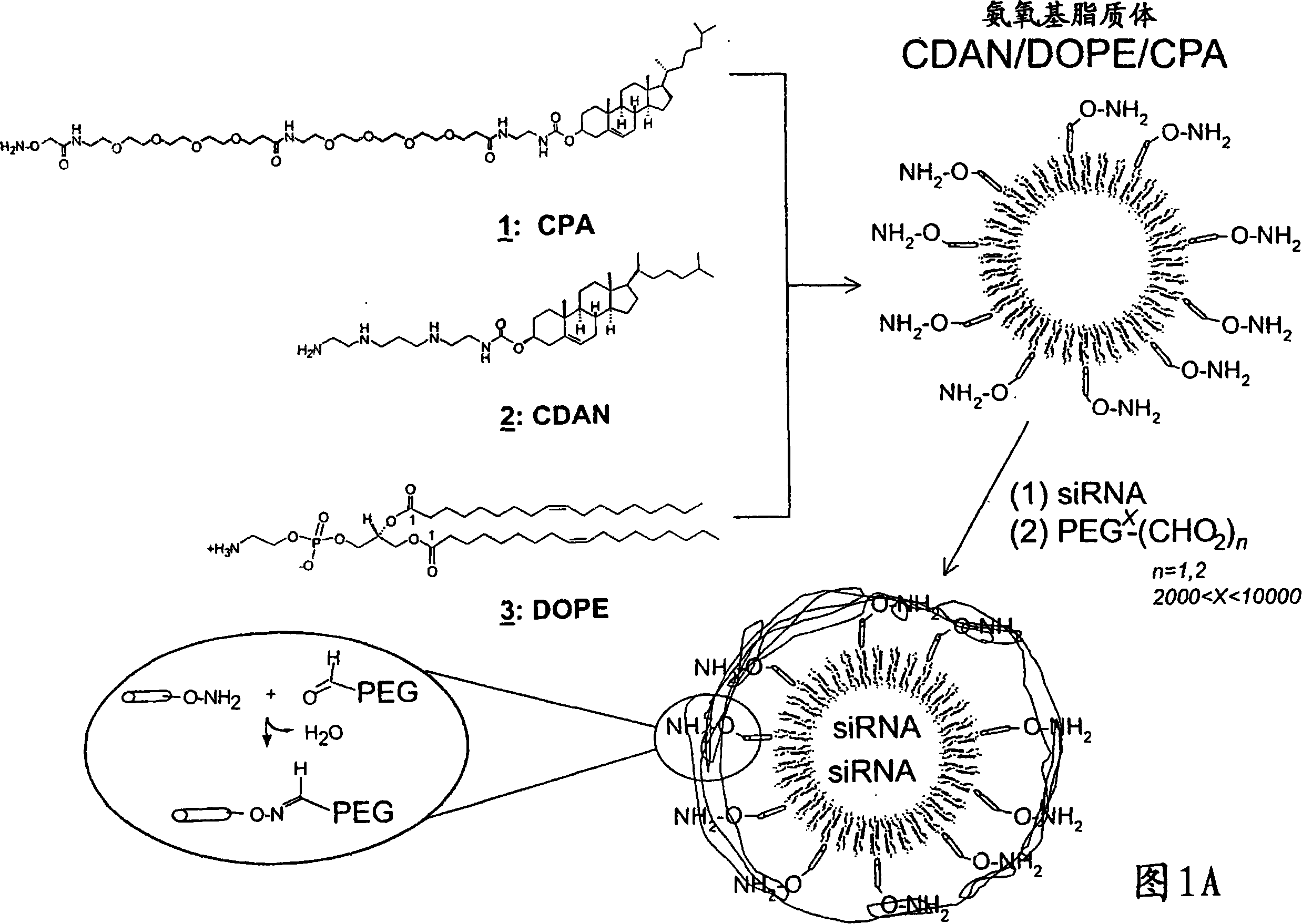
Carrier
A carrier and delivery carrier technology, applied in the field of targeted non-viral delivery vectors, can solve the problems that basic research has not yet been reported, and siRNA delivery is at a primary level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0449] PEGylated siRNA-lipoplex
[0450] Referring to accompanying drawing IA, DOPE (140 μ L, in CHCl 3 10.68mg / mL in CHCl), CDAN·3HCl (271μL, in CHCl 3 4mg / mL) and CPA (100μL, in CHCl 3 4.4 mg / mL) in a round-bottomed flask (5 mL) pretreated with nitric acid and dimethylsilyl dichloride, and the solvent was removed under reduced pressure to form a lipid film by adding water under vortexing (milliQ, 1 mL) to rehydrate. Then in the Sonomatic TM Unilamellar liposomes (SUV) were obtained by sonicating the multilamellar liposome formulation for 30 minutes in a water bath (Longford Ultrasonics). 250 μL of these liposomes were pipetted into 5 mL Falcon plastic tubes and siRNA solution (0.28 mg / mL) was added dropwise under vigorous vortexing, followed by polyethylene glycol-bisaldehyde (Mw 3400, 7.8 μL, 10 mg / mL; 5% PEG / total lipid). Samples were kept stable for 15 min / RT after which time PBS (483 μL) was added. After keeping the samples for 16 hours / RT, the volume was reduced ...
Embodiment 2
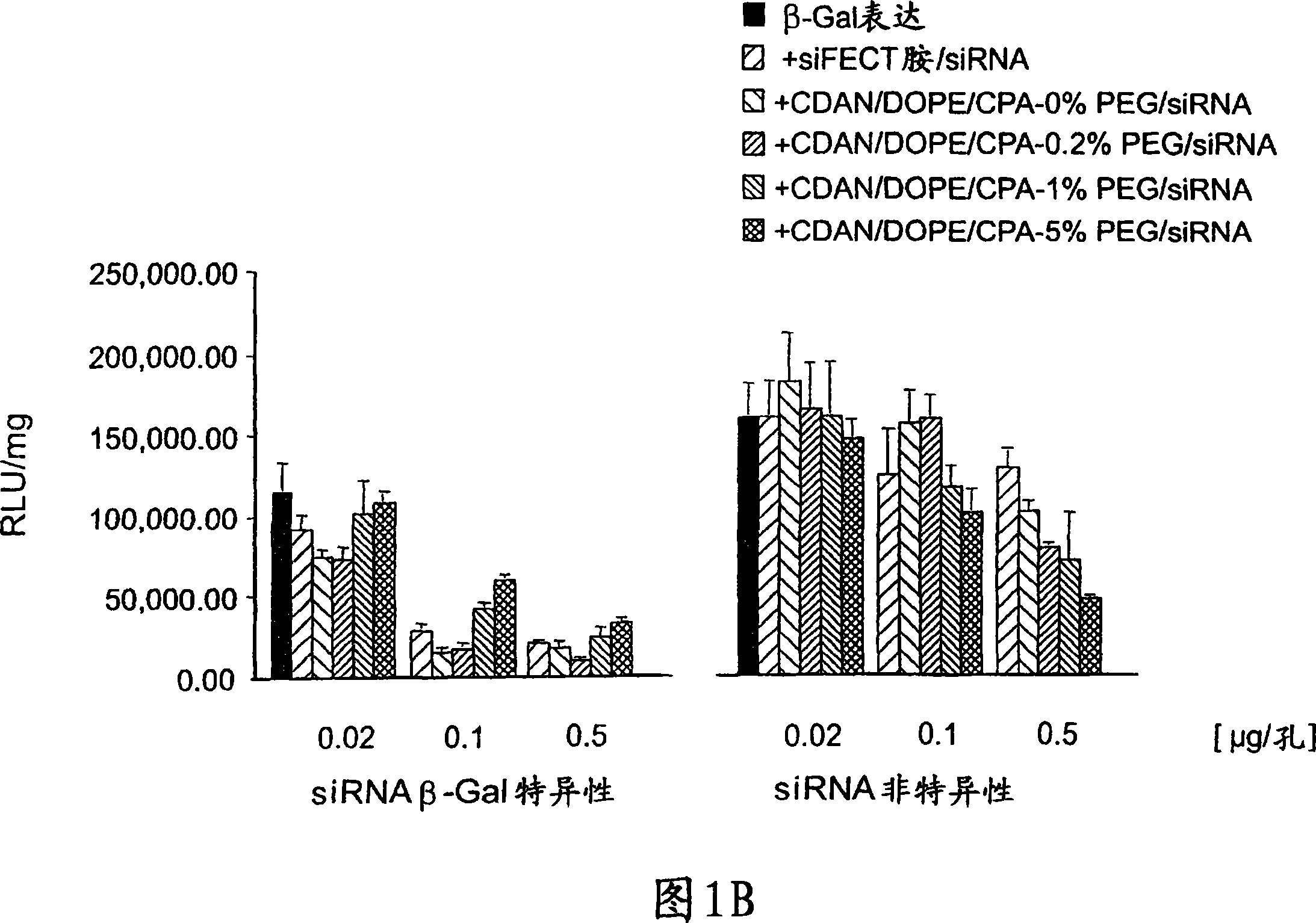
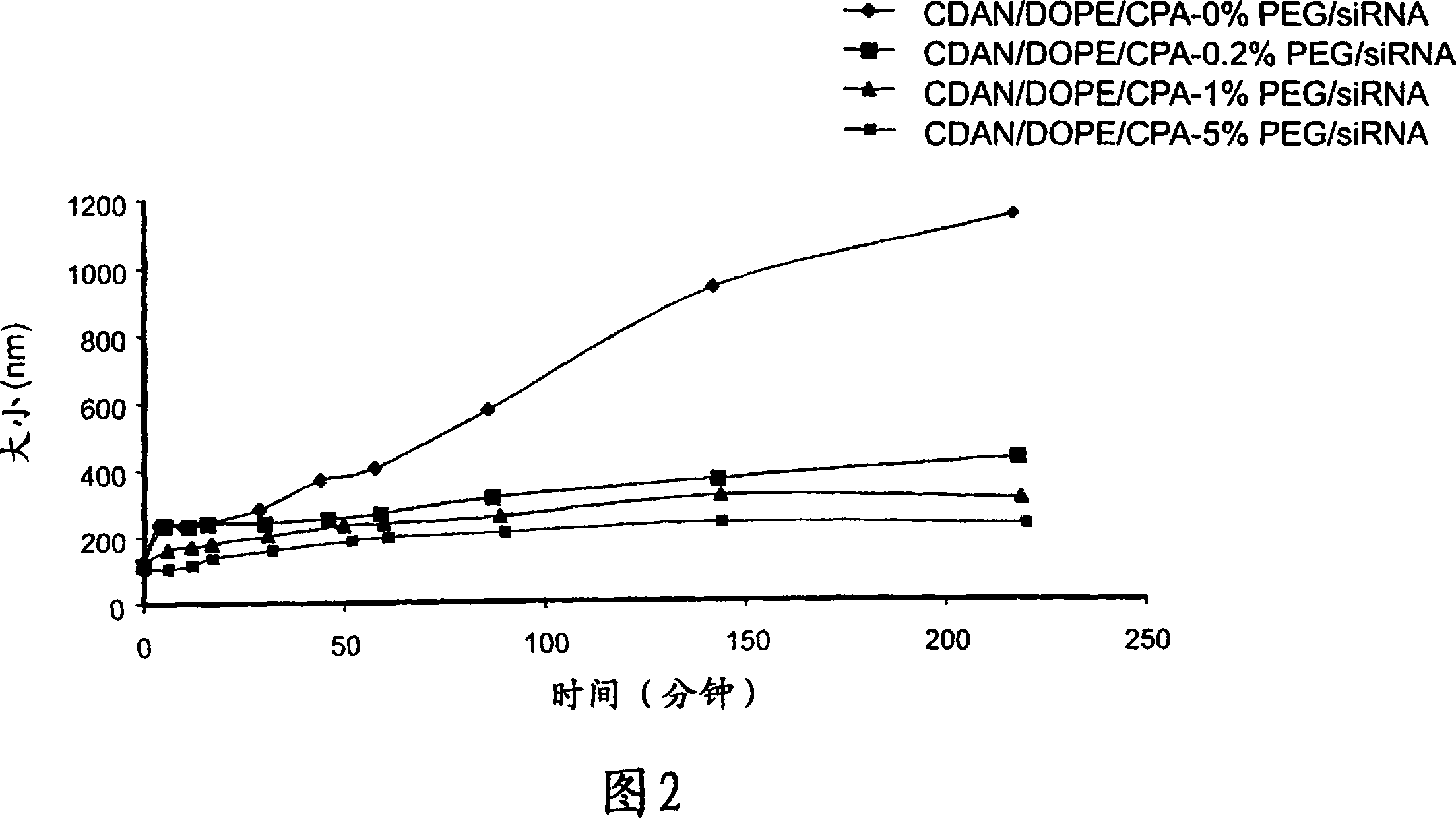
[0453] Stability of PEGylated siRNA-lipoplex.
[0454] The stability of PEGylated (Mw 2000) siRNA-lipoplex in 80% serum (FCS) was studied. Surface PEGylated LsiR complexes were generated as described in Example 1 and Figure 1 and incubated with FCS for various times, after which particle size was determined by photon correlation spectroscopy (PCS). The results are shown in Figure 2. As the degree of PEG increases, particle size does not increase over the timescale studied.
[0455] Advantageously, PEGylated siRNA-lipoplexes produced by coupling PEG to siRNA-loaded lipoplexes exhibit serum stability with increasing amounts of PEG coupled to the surface.
Embodiment 3
[0457] Tissue distribution studies
[0458] Using lipids [4- 14 C] Cholesterol (Amersham Biosciences) CDAN / DOPE / CPA700 / [4- 14 C] Cholesterol-labeled liposome formulation. siRNA lipoplexes were prepared by adding siRNA to liposomes at a liposome / siRNA ratio of 13:1 (w / w) under vortexing. Samples were concentrated to half of the total volume and replenished to the original volume by adding PBS. 200 μL (0.1 mg / mL siRNA) of these complexes were injected into the caudal vein of each mouse weighing approximately 30 g. Radioactivity was adjusted to approximately 0.035 μCI / animal.
[0459] After 1 hour, mice were anesthetized and blood was obtained by cardiac puncture and immediately mixed with 15 U heparin. Blood concentrations of liposomes were calculated assuming a total blood weight of 6% of body weight. After cervical dislocation, liver, spleen, kidney, lung and heart were dissected and weighed. Homogenize the organs in PBS at a concentration of 5 mL PBS / g organ. Aliquots...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com